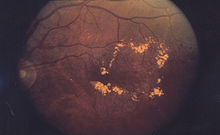
Macular edema
Cystoid macular edema (CME) involves fluid accumulation in the outer plexiform layer secondary to abnormal perifoveal retinal capillary permeability.
The cause for CME can be remembered with the mnemonic "DEPRIVEN" (diabetes, epinepherine, pars planitis, retinitis pigmentosa, Irvine-Gass syndrome, venous occlusion, E2-prostaglandin analogues, nicotinic acid/niacin).
[13] Iluvien, a sustained release intravitreal implant developed by Alimera Sciences, has been approved in Austria, Portugal and the U.K. for the treatment of vision impairment associated with chronic diabetic macular edema (DME) considered insufficiently responsive to available therapies.
[14] In 2013 Lucentis by intravitreal injection was approved by the National Institute for Health and Care Excellence in the UK for the treatment of macular edema caused by diabetes[15] and/or retinal vein occlusion.
[23] Anti‐tumour necrosis factor agents have been proposed as a treatment for macular oedema due to uveitis but a Cochrane Review published in 2018 found no relevant randomised controlled trials.