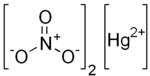
Mercury(II) nitrate
Mercury(II) nitrate is an inorganic compound with the chemical formula Hg(NO3)2.
It contains mercury(II) cations Hg2+ and nitrate anions NO−3, and water of crystallization H2O in the case of a hydrous salt.
Anhydrous and hydrous salts are colorless or white soluble crystalline solids that are occasionally used as a reagents.
[clarification needed] Mercury(II) nitrate is used as an oxidizing agent in organic synthesis, as a nitrification agent, as an analytical reagent in laboratories, in the manufacture of felt, and in the manufacture of mercury fulminate.
[2] An alternative qualitative Zeisel test can be done with the use of mercury(II) nitrate instead of silver nitrate, leading to the formation of scarlet red mercury(II) iodide.