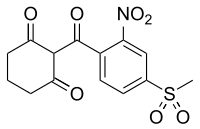
Mesotrione
[1] It is a synthetic compound inspired by the natural substance leptospermone found in the bottlebrush tree Callistemon citrinus.
[4] The invention of the triketone class of herbicides had its beginnings in an observation in 1977 of allelopathic weed control near a bottlebrush tree, Callistemon citrinus.
Chemists at the Stauffer Chemical Company identified the compound responsible as leptospermone, a known natural product[5] which had not previously been reported as having biological activity.
[6][7][8] The triketone herbicides were found to be effective on a wide range of commercially-important weed species and to have both pre- and post-emergence activity.
It achieves this selectivity and lack of damage to the crop owing to its greater potency on the target enzyme found in dicotyledons than monocotyledons[10] and because maize can metabolise the compound in the dione-containing ring.
[14] Mesotrione possesses a broad spectrum of activity on commercially important broadleaf weeds including Abutilon theophrasti, Amaranthus powellii, Amaranthus retroflexus, Chenopodium album, Datura stramonium, Digitaria sanguinalis, Lamium purpureum, Polygonum persicaria, Rumex crispus, Senecio vulgaris, Solanum nigrum, Stellaria media and Xanthium strumarium.
[15] The estimated annual use of mesotrione in US agriculture is mapped by the US Geological Service and shows an increasing trend from its introduction in 2001 to 2018, the latest date for which figures are available and now reaching 4,500,000 pounds (2,000,000 kg).
[21] In some cases, the risks of resistance developing can be reduced by using a mixture of two or more herbicides which each have activity on relevant weeds but with unrelated mechanisms of action.
By international convention and in many countries the law, pesticide labels are required to include the common name of the active ingredients.