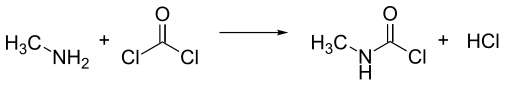
Methyl isocyanate
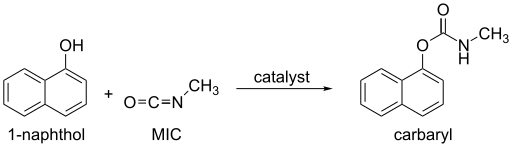
Methyl isocyanate is an intermediate chemical in the production of carbamate pesticides and Haffmann Bromamide Degradation (such as carbaryl, carbofuran, methomyl, and aldicarb).
MIC was the principal toxicant involved in the Bhopal gas disaster, which short-term killed 4,000–8,000 people and caused permanent injury and premature deaths to approximately 15,000-20,000.
The methyl isocyanate is obtained by treating the MCC with a tertiary amine, such as N,N-dimethylaniline, or with pyridine,[14] or by separating it by using distillation techniques.
With water, it forms 1,3-dimethylurea and carbon dioxide with the evolution of heat (1358.5 joules, or 325 calories, per gram of MIC): It is relatively slow to react at below 68 °F, but will increase its rate with elevated temperatures or in the presence of acid or base.
[12] Alcohols and phenols, which contain an O-H group, react slowly with MIC, but the reaction can be catalyzed by trialkylamines or dialkyltin dicarboxylate.
Exposure symptoms include coughing, chest pain, dyspnea, asthma, irritation of the eyes, nose and throat, as well as skin damage.
Higher levels of exposure, over 21 ppm, can result in pulmonary or lung edema, emphysema and hemorrhages, bronchial pneumonia and death.
[22] Proper care must be taken to store methyl isocyanate because of its ease of exothermically polymerizing (see Reactions) and its similar sensitivity to water.
[citation needed] The toxic effect of the compound was apparent in the 1984 Bhopal disaster, when around 42,000 kilograms (93,000 lb) of methyl isocyanate and other gases were released from the underground reservoirs of the Union Carbide India Limited (UCIL) factory, over a populated area on 3 December 1984, killing about 3,500 people immediately, 8,000 people in the first 48 hours and 15,000 more over the next several years.
[28] The mechanism of methyl isocyanate was previously suspected to be the carbamylation of hemoglobin, thus interfering with its oxygen-binding capability and causing hypoxia.