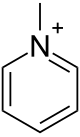
Methylpyridinium
[1] Methylpyridinium is prepared by treating pyridine with dimethylsulfate:[2] It is found in some coffee products.
[3] It is not present in unroasted coffee beans, but is formed during roasting from its precursor chemical, trigonelline.
[3] It is under investigation by scientists regarding its potential anti-carcinogenic properties,[4] particularly an effect on colon cancer.
[3] The chloride salt of N-methylpyridinium behaves as an ionic liquid.
Mixtures of that salt and zinc chloride have been characterised over the temperature range 150–200 °C (423–473 K).