Moderna COVID-19 vaccine
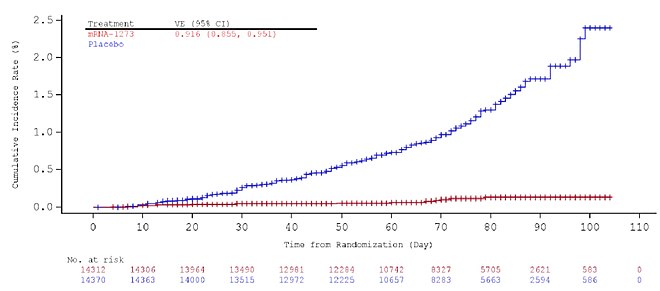
Clinical trials and real-world data have demonstrated the vaccine's high efficacy, with significant effectiveness observed two weeks post-administration of the second dose, offering 94% protection against Covid and robust defense against severe cases.
The vaccine's longevity and continuous protection are under study, with ongoing research focusing on its duration of effectiveness, which remains partially undetermined as of the latest updates.
The safety profile of the vaccine is favorable, with common side effects including injection site pain, fatigue, and headaches.
The vaccine's formulation utilizes mRNA technology, encapsulated within lipid nanoparticles to ensure cellular uptake and immune system response.
[87] Real-world observations through the CDC v-safe tracking program have not uncovered unusual numbers of adverse events or outcomes of interest.
[70] The US Centers for Disease Control and Prevention (CDC) has reported anaphylaxis (a severe allergic reaction) in 2.5 cases per million doses administered and has recommended a 15-minute observation period after injection.
[92] Delayed cutaneous reactions at injection sites resulting in rash-like erythemas have also been observed in rare cases but are not considered serious or contraindications to subsequent vaccination.
[41] In June 2021, the US CDC confirmed that myocarditis or pericarditis occurs in about 13 of every 1 million young people, mostly male and over the age of 16, who received the Moderna or the Pfizer–BioNTech vaccine.
The vaccine encodes a version of the spike protein with a modification called 2P, in which the protein includes two stabilizing mutations in which the original amino acids are replaced with prolines, developed by researchers at the University of Texas at Austin and the National Institute of Allergy and Infectious Diseases' Vaccine Research Center.
The first step of the process—synthesis of DNA plasmids (to be used as a template for synthesis of mRNA)—has been handled by a contractor called Aldevron based in Fargo, North Dakota.
[106] For the remainder of the process, Moderna contracted with Lonza Group to manufacture the vaccine at facilities in Portsmouth, New Hampshire in the United States, and in Visp in Switzerland, and purchased the necessary lipid excipients from CordenPharma.
[111] Later that month, Moderna announced its plans to spend billions of dollars to boost production of its vaccines, potentially tripling the output in 2022, claiming as well that it would make no less than 800 million doses in 2021.
[123][124][125] Moderna received US$955 million from the Biomedical Advanced Research and Development Authority (BARDA), an office of the US Department of Health and Human Services.
[132] Moderna signed a partnership with Swiss vaccine manufacturer Lonza Group,[133] to supply 300 million doses per annum.
[134] In May 2020, Moderna began a phase IIa clinical trial recruiting six hundred adult participants to assess safety and differences in antibody response to two doses of its candidate vaccine, mRNA-1273, a study expected to complete in 2021.
[135] In July 2020, Moderna scientists published preliminary results of the phase I dose escalation clinical trial of mRNA-1273, showing dose-dependent induction of neutralizing antibodies against S1/S2 as early as 15 days post-injection.
Mild to moderate adverse reactions, such as fever, fatigue, headache, muscle ache, and pain at the injection site were observed in all dose groups, but were common with increased dosage.
[104] In July 2020, Moderna announced in a preliminary report that its Operation Warp Speed candidate had led to production of neutralizing antibodies in healthy adults in phase I clinical testing.
The study also found that cross-reactive T cells acquired during infection with other coronaviruses that cause the common cold increased the response to the vaccine.
[146] In February 2021, results from phase III clinical trial were published in the New England Journal of Medicine, indicating 94% efficacy in preventing COVID‑19 infection.
[172] In August 2021, Malaysia's National Pharmaceutical Regulatory Agency (NPRA) gave conditional registration for emergency use of the Moderna COVID‑19 vaccine.
[176][177][178][179][180][181] In March 2021, the Medicines and Healthcare products Regulatory Agency (MHRA) granted conditional marketing authorization in the United Kingdom.
[186] In August 2021, the US Food and Drug Administration (FDA) and the US Centers for Disease Control and Prevention (CDC) authorized the use of an additional mRNA vaccine dose for immunocompromised individuals.
[69] In October 2021, the US Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) authorized the use of either homologous or heterologous vaccine booster doses.
[193] In January 2021, Moderna started development of a new form of its vaccine, called mRNA-1273.351, that could be used as a booster shot against the Beta variant (lineage B.1.351).
[195][186] In February 2021, Moderna announced that it had manufactured and shipped sufficient amounts of mRNA-1273.351 to the National Institutes of Health to run phase I clinical trials.
[208][209] In September 2024, the UK's Medicines and Healthcare products Regulatory Agency (MHRA) approved Moderna's JN.1-adapted COVID-19 vaccine for use in adults and children aged six months and older.
[237][238][239][240][241] In May 2020, after releasing partial and non-peer-reviewed results for only eight of 45 candidates in a preliminary pre-phase I stage human trial directly to financial markets, the CEO announced on CNBC an immediate $1.25 billion rights issue to raise funds for the company, at a $30 billion valuation,[242] while Stat said, "Vaccine experts say Moderna didn't produce data critical to assessing COVID‑19 vaccine.
[246] Scientists and the WHO reaffirmed the lack of evidence on the need for a booster dose for healthy people and that the vaccine remains effective against severe disease months after administration.
[250][251][252][253] In November 2021, a White House correspondent for the conservative outlet Newsmax falsely tweeted that the Moderna vaccine contained luciferase "so that you can be tracked.