Monazite
Due to the alpha decay of thorium and uranium, monazite contains a significant amount of helium, which can be extracted by heating.
The M(III) centers have a distorted coordination sphere being surrounded by eight oxides with M–O distances around 2.6 Å in length.
Monazite sand was also briefly mined in North Carolina, but, shortly thereafter, extensive deposits in southern India were found.
Brazilian and Indian monazite dominated the industry before World War II, after which major mining activity transferred to South Africa.
[citation needed] Because of their high density, monazite minerals concentrate in alluvial sands when released by the weathering of pegmatites.
Typically, the lanthanides in such monazites contain about 45–48% cerium, about 24% lanthanum, about 17% neodymium, about 5% praseodymium, and minor quantities of samarium, gadolinium, and yttrium.
Very low concentrations of the heaviest lanthanides in monazite justified the term "rare" earth for these elements, with prices to match.
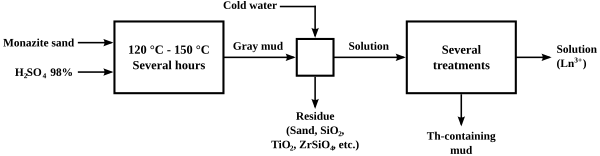
The original process for "cracking" monazite so as to extract the thorium and lanthanide content was to heat it with concentrated sulfuric acid to temperatures between 120 and 150 °C (250 and 300 °F) for several hours.
Products of the uranium and thorium decay series, particularly radium will be present in trace amounts and form a radiotoxic hazard.
While radium-228 (a product of thorium decay) will be present only in extremely minute amounts (less than one milligram per metric ton of thorium), and will decay away with a half life of roughly 5.75 years, radium-226 will be present at a ratio above 300 milligrams per metric ton of uranium and due to its long half life (~1600 years) will essentially remain with the residue.
One study done at Oak Ridge National Laboratory in Tennessee[16] the performance of synthetic monazite to borosilicate glass in radioactive waste management is compared.
This experiment involved synthetic monazite and borosilicate glass being soaked in a contaminated simulated Savannah River defense wastes for 28 days, during the time period the leaching rates from both materials were measured.
Synthetic monazite is also shown to have similar durability to that of the natural crystalline samples after it becomes fully amorphized.