Neuroimmune system
[6] However, during a neuroimmune response, certain peripheral immune cells are able to cross various blood or fluid–brain barriers in order to respond to pathogens that have entered the brain.
[12] The neuroimmune system uses complementary processes of both sensory neurons and immune cells to detect and respond to noxious or harmful stimuli.
[11] For example, invading bacteria may simultaneously activate inflammasomes, which process interleukins (IL-1 β), and depolarize sensory neurons through the secretion of hemolysins.
[11] This causes the combined response of both a resulting action potential due to the depolarization created by the influx of calcium and potassium ions, and the activation of inflammasomes.
[7] For example, the chemokine fractalkine has been implicated in communication between microglia and dorsal root ganglion (DRG) neurons in the spinal cord.
[15] Following apoptosis, dead neurons secrete chemical signals that bind to microglial cells and cause them to devour harmful debris from the surrounding nervous tissue.
[11] When the action potential travels back down the spinal nerve network, another impulse travels to peripheral sensory neurons that secrete amino acids and neuropeptides like calcitonin gene-related peptide (CGRP) and Substance P.[11][13] These chemicals act by increasing the redness, swelling of damaged tissues, and attachment of immune cells to endothelial tissue, thereby increasing the permeability of immune cells across capillaries.
Nociceptors are also associated with the body's reflexes to pathogens as they are in strategic locations, such as airways and intestinal tissues, to induce muscle contractions that cause scratching, vomiting, and coughing.
[11] In terms of intestinal and bronchial parasites, vomiting, coughing, sneezing, and diarrhea can also be caused by nociceptor stimulation in infected tissues, and nerve impulses originating from the brain stem that innervate respective smooth muscles.
[18] In both cases, the release of eosinophils and other immune molecules cause a hypersensitization of sensory neurons in bronchial airways that produce enhanced symptoms.
[11][18] It has also been reported that increased immune cell secretions of neurotrophins in response to pollutants and irritants can restructure the peripheral network of nerves in the airways to allow for a more primed state for sensory neurons.
Studies, in animals, indicate that psychological stress raises glucocorticoid levels and eventually, an increase in susceptibility to streptococcal skin infections.
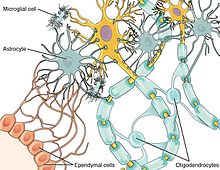
[15] This demyelinating effect is a result of the secretion of cytokines and matrix metalloproteinases (MMP) from activated astrocyte cells onto neighboring neurons.
[15] Astrocytes that remain in an activated state form glial scars that also prevent the re-myelination of neurons, as they are a physical impediment to oligodendrocyte progenitor cells (OPCs).
[23] The neuroimmune system is also involved in asthma and chronic cough, as both are a result of the hypersensitized state of sensory neurons due to the release of immune molecules and positive feedback mechanisms.