Nickel(II) nitrate
Nickel nitrate is the inorganic compound Ni(NO3)2 or any hydrate thereof.
Rather it is generated by the reaction of hydrates with dinitrogen pentoxide or of nickel carbonyl with dinitrogen tetroxide:[3] The hydrated nitrate is often used as a precursor to supported nickel catalysts.
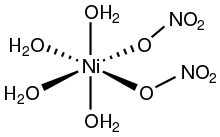
[3] Nickel(II) compounds with oxygenated ligands often feature octahedral coordination geometry.
In heterogeneous catalysis, nickel(II) nitrate is used to impregnate alumina.
[6] In homogeneous catalysis, the hexahydrate is a precatalyst for cross coupling reactions.