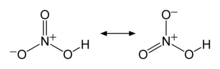
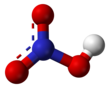
Nitric acid
[6] The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into oxides of nitrogen.
Nitric acid is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule.
[7] The conventional view is that nitric acid was first described in pseudo-Geber's De inventione veritatis ("On the Discovery of Truth", after c. 1300).
[8] However, according to Eric John Holmyard and Ahmad Y. al-Hassan, the nitric acid also occurs in various earlier Arabic works such as the Ṣundūq al-ḥikma ("Chest of Wisdom") attributed to Jabir ibn Hayyan (8th century) or the Taʿwīdh al-Ḥākim attributed to the Fatimid caliph al-Hakim bi-Amr Allah (985–1021).
There will flow down by reason of the heat an oil like cow's butter.Nitric acid is also found in post-1300 works falsely attributed to Albert the Great and Ramon Llull (both 13th century).
These works describe the distillation of a mixture containing niter and green vitriol, which they call "eau forte" (aqua fortis).
"[15][a] In 1785 Henry Cavendish determined its precise composition and showed that it could be synthesized by passing a stream of electric sparks through moist air.
[12] In 1806, Humphry Davy reported the results of extensive distilled water electrolysis experiments concluding that nitric acid was produced at the anode from dissolved atmospheric nitrogen gas.
About 20% of the produced oxides of nitrogen remained unreacted so the final towers contained an alkali solution to neutralize the rest.
His method produced nitric acid from electrolysis of calcium nitrate converted by bacteria from nitrogenous matter in peat bogs.
An earthenware pot surrounded by limestone was sunk into the peat and staked with tarred lumber to make a compartment for the carbon anode around which the nitric acid is formed.
The nitrogen dioxide (NO2) and/or dinitrogen tetroxide (N2O4) remains dissolved in the nitric acid coloring it yellow or even red at higher temperatures.
Anhydrous nitric acid is a colorless, low-viscosity (mobile) liquid with a density of 1.512–3 g/cm3 that solidifies at −42 °C (−44 °F) to form white crystals.
Magnesium, manganese, and zinc liberate H2: Nitric acid can oxidize non-active metals such as copper and silver.
Nitric acid is used as a cheap means in jewelry shops to quickly spot low-gold alloys (< 14 karats) and to rapidly assess the gold purity.
As a general rule, oxidizing reactions occur primarily with the concentrated acid, favoring the formation of nitrogen dioxide (NO2).
[30][full citation needed] Metals that are passivated by concentrated nitric acid are iron, cobalt, chromium, nickel, and aluminium.
Nitration of organic compounds with nitric acid is the primary method of synthesis of many common explosives, such as nitroglycerin and trinitrotoluene (TNT).
Respective local skin color changes are indicative of inadequate safety precautions when handling nitric acid.
The combined Ostwald and Haber processes are extremely efficient, requiring only air and natural gas feedstocks.
[34] The Ostwald process' technical innovation is the proper conditions under which anhydrous ammonia burns to nitric oxide (NO) instead of dinitrogen (N2).
[36] Alternatively, thermal decomposition of copper(II) nitrate gives nitrogen dioxide and oxygen gases; these are then passed through water or hydrogen peroxide[38] as in the Ostwald process: The main industrial use of nitric acid is for the production of fertilizers.
[40] IRFNA (inhibited red fuming nitric acid) was one of three liquid fuel components for the BOMARC missile.
In elemental analysis by ICP-MS, ICP-AES, GFAA, and Flame AA, dilute nitric acid (0.5–5.0%) is used as a matrix compound for determining metal traces in solutions.
In electrochemistry, nitric acid is used as a chemical doping agent for organic semiconductors, and in purification processes for raw carbon nanotubes.
[43] The corrosive effects of nitric acid are exploited for some specialty applications, such as etching in printmaking, pickling stainless steel or cleaning silicon wafers in electronics.
[48] Nitric acid plays a key role in PUREX and other nuclear fuel reprocessing methods, where it can dissolve many different actinides.
The major hazard posed by it is chemical burns, as it carries out acid hydrolysis with proteins (amide) and fats (ester), which consequently decomposes living tissue (e.g. skin and flesh).
[50] The standard first-aid treatment for acid spills on the skin is, as for other corrosive agents, irrigation with large quantities of water.
Washing is continued for at least 10–15 minutes to cool the tissue surrounding the acid burn and to prevent secondary damage.