Non-competitive inhibition
During his research in the hospital, he was the first to view the different types of inhibition; specifically using fructose and glucose as inhibitors of maltase activity.
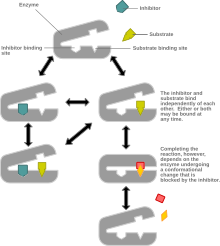
Non-competitive inhibition affects the kcat value (but not the Km) on any given graph; this inhibitor binds to a site that has specificity for the certain molecule.
[2] Like many other scientists of their time, Leonor Michaelis and Maud Menten worked on a reaction that was used to change the composition of sucrose and make it lyse into two products – fructose and glucose.
[2][3] Adrian John Brown and Victor Henri laid the groundwork for the discoveries in enzyme kinetics that Michaelis and Menten are known for.
[4] Brown theoretically envisioned the mechanism now accepted for enzyme kinetics, but did not have the quantitative data to make a claim.
[4] Victor Henri made significant contributions to enzyme kinetics during his doctoral thesis, however he lacked noting the importance of hydrogen ion concentration and mutarotation of glucose.
The goal of Henri's thesis was to compare his knowledge of enzyme-catalysed reactions to the recognized laws of physical chemistry.
Using glucose and fructose in the catalytic reactions controlled by maltase and invertase, Leonor Michaelis was the first scientist to distinguish the different types of inhibition by using the pH scale which did not exist in Henri's time.
This type of inhibition reduces the maximum rate of a chemical reaction without changing the apparent binding affinity of the catalyst for the substrate (Kmapp – see Michaelis-Menten kinetics).
In non-competitive inhibition, the inhibitor binds to an allosteric site and prevents the enzyme-substrate complex from performing a chemical reaction.
This can be seen as a consequence of Le Chatelier's principle because the inhibitor binds to both the enzyme and the enzyme-substrate complex equally so that the equilibrium is maintained.