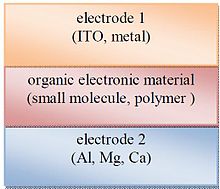
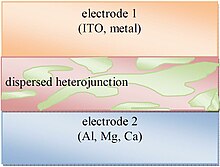
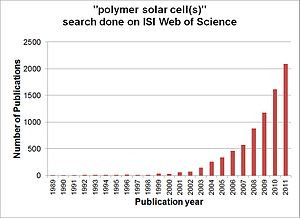
Organic solar cell
The molecules used in organic solar cells are solution-processable at high throughput and are cheap, resulting in low production costs to fabricate a large volume.
Depending on the band gap of the light-absorbing material, photovoltaic cells can also convert low-energy, infrared (IR) or high-energy, ultraviolet (UV) photons into DC electricity.
Unlike in an inorganic crystalline PV cell material, with its band structure and delocalized electrons, excitons in organic photovoltaics are strongly bound with an energy between 0.1 and 1.4 eV.
[12][13][14] In 1958 the photovoltaic effect or the creation of voltage of a cell based on magnesium phthalocyanine (MgPc)—a macrocyclic compound having an alternating nitrogen atom-carbon atom ring structure—was discovered to have a photovoltage of 200 mV.
The materials are chosen to make the differences large enough that these local electric fields are strong, which splits excitons much more efficiently than single layer photovoltaic cells.
The domain sizes of this blend are on the order of nanometers, allowing for excitons with short lifetimes to reach an interface and dissociate due to the large donor-acceptor interfacial area.
A blend of CN-PPV and MEH-PPV with Al and ITO as the electrodes, yielded peak monochromatic power conversion efficiency of 1% and fill factor of 0.38.
The thickness of the generated film affects the phases segregation because the dynamics of crystallization and precipitation are different for more concentrated solutions or faster evaporation rates (needed to build thicker devices).
[46] The gradients in the initial morphology are then mainly generated by the solvent evaporation rate and the differences in solubility between the donor and acceptor inside the blend.
In 2009 the difference in vertical distribution on P3HT:PCBM solar cells was shown to cause problems with electron mobility which ends up with the yielding of very poor device efficiencies.
This "line of sight" technique also can create holes in the film due to shadowing, which causes an increase in the device series-resistance and short circuit.
Depending on the growth parameters (temperature of the source, base pressure and flux of the carrier gas) the deposited film can be crystalline or amorphous in nature.
This morphology originates from the liquid-liquid phase separation during drying; solve evaporation causes the mixture to enter into the spinodal region, in which there are significant thermal fluctuations.
[83] When the top electrode is made transparent, the cell's ability to trap the electromagnetic field in the absorber layer decreases, resulting in a low PCE.
[84] In 2014, near-infrared polymer solar cells based on a copolymer of naphthodithiophene diimide and bithiophene (PNDTI-BT-DT) were fabricated in combination with PTB7 as an electron donor.
The researchers proposed a semi-transparent PSC with enhanced efficiency that utilizes both narrow bandgap polymer donor, PTB7-Th, and non-fullerene acceptor, IHIC.
PCE (η) is proportional to the product of the short-circuit current (JSC), the open-circuit voltage (VOC), and the fill factor (FF), all of which can be determined from a current-voltage curve.
Recent advances in polymer solar cell performance have resulted from compressing the bandgap to enhance short-circuit current while lowering the Highest Occupied Molecular Orbital (HOMO) to increase open-circuit voltage.
[90] However, efforts are being made to upscale manufacturing of polymer solar cells, in order to decrease costs and also advocate for a practical approach for PSC production.
However, roll-to-roll solution processing is ill-suited for on-grid electricity production due to the short lifetime of polymer solar cells.
[94] Designing organic solar cells requires optimization of a large number of structural and compositional parameters, such as band gaps and layer thicknesses.
[97] Due to having low mobility, efficient bulk heterojunction photovoltaics have to be designed with thin active layers to avoid recombination of the charge carriers, which is detrimental to absorption and scalability in processing.
Whilst traveling to the electrode, a charge can become trapped and/or recombine in a disordered interpenetrating organic material, resulting in decreased device efficiency.
These attributes are not only influenced by the molecular structure but are also quite sensitive to processing conditions, making the study of mechanical properties of polymer thin films such as tensile modulus, ductility and fracture toughness under strain rather difficult.
Various studies have related the cohesive or adhesive fracture energy Gc , defined as the work required to break separate polymer interfaces to molecular parameters and processing conditions.
While fullerene acceptors have been the standard for most organic photovoltaics due to their compatibility within bulk heterojunction cell designs as well as their good transport properties, they do have some fallbacks that are leading researchers to attempt to find alternatives.
[117] Combining a polymer donor (D18) with a small molecule acceptor (Y6), scientists have fabricated organic solar cells in the laboratory giving high efficiencies over 18%.
Appending alkyl chains to NFAs has led to increases in solubility but decreases in molecular packing (π-stacking), which leads to no net impact on PCE.
A guest molecule named BTO with oligo(ethylene glycol) (OEG) side chains used in conjunction with the NFA Y6 as the acceptor, PM6 as the donor, and paraxylene (PX) as the high-melting-point and sustainable solvent led to an increase in PCE from 11% to over 16%, regarded an acceptable level of efficiency.
Since small molecules do not vary in molecular weights the way polymers do, they would require less purification steps and are less susceptible to macromolecule defects and kinks that can create trap states leading to recombination.