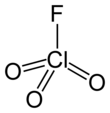
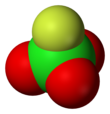
Perchloryl fluoride
[3]: 380 It is quite reactive towards reducing agents and anions, however, with the chlorine atom acting as an electrophile.
[8] A common fluorinator in modern syntheses is antimony pentafluoride:[3]: 372–373 Alternatively, potassium perchlorate reacts with excess fluorosulfuric acid to give potassium bisulfate and perchloryl fluoride:[8] ClO3F reacts with alcohols to produce alkyl perchlorates, which are extremely shock-sensitive explosives.
[11] Perchloryl fluoride was investigated as a high performance liquid rocket fuel oxidizer.
Rocket fuel chemist John Drury Clark reported in his book Ignition!
Exposure to toxic levels causes severe respiratory tract inflammation and pulmonary edema.