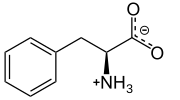
Phenylalanine
This essential amino acid is classified as neutral, and nonpolar because of the inert and hydrophobic nature of the benzyl side chain.
Phenylalanine is a precursor for tyrosine, the monoamine neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), and the biological pigment melanin.
[4] The first description of phenylalanine was made in 1879, when Schulze and Barbieri identified a compound with the empirical formula, C9H11NO2, in yellow lupine (Lupinus luteus) seedlings.
This discovery helped to establish the nature of the coding relationship that links information stored in genomic nucleic acid with protein expression in the living cell.
[8] The Food and Nutrition Board (FNB) of the U.S. Institute of Medicine set Recommended Dietary Allowances (RDAs) for essential amino acids in 2002.
In excessive quantities, supplementation can interfere with the production of serotonin and other aromatic amino acids[11] as well as nitric oxide due to the overuse (eventually, limited availability) of the associated cofactors, iron or tetrahydrobiopterin.
[citation needed] The corresponding enzymes for those compounds are the aromatic amino acid hydroxylase family and nitric oxide synthase.
Pregnant women with hyperphenylalaninemia may show similar symptoms of the disorder (high levels of phenylalanine in blood), but these indicators will usually disappear at the end of gestation.
The stereoisomer D-phenylalanine (DPA) can be produced by conventional organic synthesis, either as a single enantiomer or as a component of the racemic mixture.
[17] DL-Phenylalanine (DLPA) is marketed as a nutritional supplement for its purported analgesic and antidepressant activities, which have been supported by clinical trials.
It appears to cross the blood–brain barrier less efficiently than L-phenylalanine, and so a small amount of an ingested dose of D-phenylalanine is excreted in the urine without penetrating the central nervous system.
[29] L-Phenylalanine also inhibits neurotransmitter release at glutamatergic synapses in hippocampus and cortex with IC50 of 980 μM, a brain concentration seen in classical phenylketonuria, whereas D-phenylalanine has a significantly smaller effect.
The quantity of L-phenylalanine produced commercially has been increased by genetically engineering E. coli, such as by altering the regulatory promoters or amplifying the number of genes controlling enzymes responsible for the synthesis of the amino acid.