Phenyllithium
The primary use of PhLi is to facilitate formation of carbon-carbon bonds by nucleophilic addition and substitution reactions: 2-Phenylpyridine is prepared by the reaction of phenyl lithium with pyridine, a process that entails an addition-elimination pathway:[6] Phenyllithium is an organolithium compound that forms monoclinic crystals.
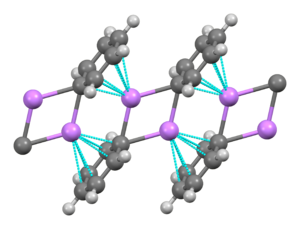
[7] In solution, it takes a variety of structures dependent on the organic solvent.
Four Li atoms and four ipso carbon centers occupy alternating vertices of a distorted cube.
Phenyl groups are at the faces of the tetrahedron and bind to three of the nearest Li atoms.
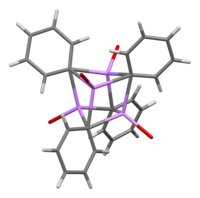
In the presence of LiBr, a byproduct of directly reacting lithium with a phenyl halide, the [(PhLi·Et2O)4] complex instead becomes [(PhLi·Et2O)3·LiBr].