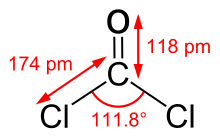
Phosgene
It is a toxic, colorless gas; in low concentrations, its musty odor resembles that of freshly cut hay or grass.
This involves maintaining equivalent rates of production and consumption, which keeps the amount of phosgene in the system at any one time fairly low, reducing the risks in the event of an accident.
[11] Simple organochlorides slowly convert into phosgene when exposed to ultraviolet (UV) irradiation in the presence of oxygen.
[12] Before the discovery of the ozone hole in the late 1970s large quantities of organochlorides were routinely used by industry, which inevitably led to them entering the atmosphere.
[14] Less than 1% makes it to the stratosphere, where it is expected to have a lifetime of several years, since this layer is much drier and phosgene decomposes slowly through UV photolysis.
[17] Phosgene was synthesized by the Cornish chemist John Davy (1790–1868) in 1812 by exposing a mixture of carbon monoxide and chlorine to sunlight.
The synthesis of isocyanates from amines illustrates the electrophilic character of this reagent and its use in introducing the equivalent synthon "CO2+":[19] Such reactions are conducted on laboratory scale in the presence of a base such as pyridine that neutralizes the hydrogen chloride side-product.
[21] Phosgene reacts with water to release hydrogen chloride and carbon dioxide: Analogously, upon contact with ammonia, it converts to urea: Halide exchange with nitrogen trifluoride and aluminium tribromide gives COF2 and COBr2, respectively.
[9] It is listed on Schedule 3 of the Chemical Weapons Convention: All production sites manufacturing more than 30 tonnes per year must be declared to the OPCW.
[31] At low concentrations, phosgene may have a pleasant odor of freshly mown hay or green corn,[32] but has also been described as sweet, like rotten banana peels.
[33][34] Therefore, persons in workplaces where there exists risk of accidental phosgene release usually wear indicator badges close to the nose and mouth.
[35] In case of low or moderate quantities of inhaled phosgene, the exposed person is to be monitored and subjected to precautionary therapy, then released after several hours.
For higher doses of inhaled phosgene (above 150 ppm × min) a pulmonary edema often develops which can be detected by X-ray imaging and regressive blood oxygen concentration.
The risk connected to a phosgene inhalation is based not so much on its toxicity (which is much lower in comparison to modern chemical weapons like sarin or tabun) but rather on its typical effects: the affected person may not develop any symptoms for hours until an edema appears, at which point it could be too late for medical treatment to assist.
On the other hand, pulmonary edemas treated in a timely manner usually heal in the mid- and longterm, without major consequences once some days or weeks after exposure have passed.
[37][38] Nonetheless, the detrimental health effects on pulmonary function from untreated, chronic low-level exposure to phosgene should not be ignored; although not exposed to concentrations high enough to immediately cause an edema, many synthetic chemists (e.g. Leonidas Zervas) working with the compound were reported to experience chronic respiratory health issues and eventual respiratory failure from continuous low-level exposure.