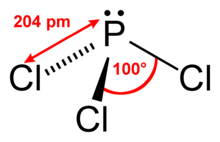
Phosphorus trichloride
A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds.
Its 31P NMR spectrum exhibits a singlet around +220 ppm with reference to a phosphoric acid standard.
It reacts with phenol to give triphenyl phosphite: Alcohols such as ethanol react similarly in the presence of a base such as a tertiary amine:[9] With one equivalent of alcohol and in the absence of base, the first product is alkoxyphosphorodichloridite:[10] In the absence of base, however, with excess alcohol, phosphorus trichloride converts to diethylphosphite:[11][12] Secondary amines (R2NH) form aminophosphines.
An industrially relevant reaction of PCl3 with amines is phosphonomethylation, which employs formaldehyde: The herbicide glyphosate is also produced this way.
Phosphorus trichloride is commonly used to convert primary and secondary alcohols to the corresponding chlorides.
[17] PCl3 is important indirectly as a precursor to PCl5, POCl3 and PSCl3, which are used in many applications, including herbicides, insecticides, plasticisers, oil additives, and flame retardants.
[18] Industrial production of phosphorus trichloride is controlled under the Chemical Weapons Convention, where it is listed in schedule 3, as it can be used to produce mustard agents.