Phosphoryl chloride
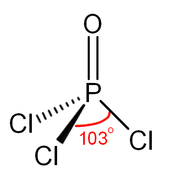
Phosphoryl chloride (commonly called phosphorus oxychloride) is a colourless liquid with the formula POCl3.
It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride.
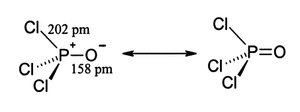
[citation needed] More modern treatments explain the tight P–O bond as a combination of lone pair transfer from the phosphorus to the oxygen atom and a dative π back-bond that produces an effective [P+]-[O−] configuration.
[9] Upon treatment with excess alcohols and phenols, POCl3 gives phosphate esters: Such reactions are often performed in the presence of an HCl acceptor such as pyridine or an amine.
Phosphoryl chloride was first reported in 1847 by the French chemist Adolphe Wurtz by reacting phosphorus pentachloride with water.
The reaction of phosphorus pentachloride with boric acid or oxalic acid:[12] Reduction of tricalcium phosphate with carbon in the presence of chlorine gas:[13] The reaction of phosphorus pentoxide with sodium chloride is also reported:[13] Phosphoryl chloride is used on an industrial scale for the manufacture of phosphate esters (organophosphates).