Planck's law
[2] At the end of the 19th century, physicists were unable to explain why the observed spectrum of black-body radiation, which by then had been accurately measured, diverged significantly at higher frequencies from that predicted by existing theories.
In 1900, German physicist Max Planck heuristically derived a formula for the observed spectrum by assuming that a hypothetical electrically charged oscillator in a cavity that contained black-body radiation could only change its energy in a minimal increment, E, that was proportional to the frequency of its associated electromagnetic wave.
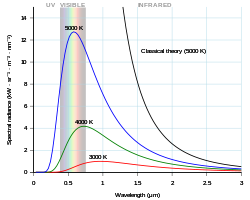
At higher temperatures, the body is bright yellow or blue-white and emits significant amounts of short wavelength radiation, including ultraviolet and even x-rays.
Nevertheless, in a manner of speaking, this formula means that the shape of the spectral distribution is independent of temperature, according to Wien's displacement law, as detailed below in § Properties §§ Percentiles.
Planck's law describes the unique and characteristic spectral distribution for electromagnetic radiation in thermodynamic equilibrium, when there is no net flow of matter or energy.
Quantum theoretical explanation of Planck's law views the radiation as a gas of massless, uncharged, bosonic particles, namely photons, in thermodynamic equilibrium.
Four decades after Kirchhoff's insight of the general principles of its existence and character, Planck's contribution was to determine the precise mathematical expression of that equilibrium distribution Bν(T).
The interface is not composed of physical matter but is a theoretical conception, a mathematical two-dimensional surface, a joint property of the two contiguous media, strictly speaking belonging to neither separately.
At any point in the interior of a black body located inside a cavity in thermodynamic equilibrium at temperature T the radiation is homogeneous, isotropic and unpolarized.
Taking into account the independence of direction of the spectral radiance of radiation from the surface of a black body in thermodynamic equilibrium, one has L0(dA, dν) = Bν(T) and so
Thus Lambert's cosine law expresses the independence of direction of the spectral radiance Bν (T) of the surface of a black body in thermodynamic equilibrium.
Also for comparison a planet modeled as a black body is shown, radiating at a nominal 288 K (15 °C) as a representative value of the Earth's highly variable temperature.
According to historian D. M. Siegel: "He was not a practitioner of the more sophisticated techniques of nineteenth-century mathematical physics; he did not even make use of the functional notation in dealing with spectral distributions.
He did not mention the possibility of ideally perfectly reflective walls; in particular he noted that highly polished real physical metals absorb very slightly.
In 1859, not knowing of Stewart's work, Gustav Robert Kirchhoff reported the coincidence of the wavelengths of spectrally resolved lines of absorption and of emission of visible light.
Planck's black bodies radiated and absorbed only by the material in their interiors; their interfaces with contiguous media were only mathematical surfaces, capable neither of absorption nor emission, but only of reflecting and transmitting with refraction.
In a series of papers from 1881 to 1886, Langley reported measurements of the spectrum of heat radiation, using diffraction gratings and prisms, and the most sensitive detectors that he could make.
Michelson published a consideration of the idea that the unknown Kirchhoff radiation function could be explained physically and stated mathematically in terms of "complete irregularity of the vibrations of ...
[88] In June of that same year, Lord Rayleigh had created a formula that would work for short lower frequency wavelengths based on the widely accepted theory of equipartition.
[64][97] On 7 October 1900, Rubens told Planck that in the complementary domain (long wavelength, low frequency), and only there, Rayleigh's 1900 formula fitted the observed data well.
On 19 October 1900, Rubens and Kurlbaum briefly reported the fit to the data,[100] and Planck added a short presentation to give a theoretical sketch to account for his formula.
[104] Planck did not attribute any definite physical significance to his hypothesis of resonant oscillators but rather proposed it as a mathematical device that enabled him to derive a single expression for the black body spectrum that matched the empirical data at all wavelengths.
[97] Partly following a heuristic method of calculation pioneered by Boltzmann for gas molecules, Planck considered the possible ways of distributing electromagnetic energy over the different modes of his hypothetical charged material oscillators.
[106] In Planck's words, "I considered the [quantum hypothesis] a purely formal assumption, and I did not give it much thought except for this: that I had obtained a positive result under any circumstances and at whatever cost.
Planck believed that in a cavity with perfectly reflecting walls and with no matter present, the electromagnetic field cannot exchange energy between frequency components.
Kuhn wrote that, in Planck's earlier papers and in his 1906 monograph,[137] there is no "mention of discontinuity, [nor] of talk of a restriction on oscillator energy, [nor of] any formula like U = nhν."
[142] The colourful term "ultraviolet catastrophe" was given by Paul Ehrenfest in 1911 to the paradoxical result that the total energy in the cavity tends to infinity when the equipartition theorem of classical statistical mechanics is (mistakenly) applied to black-body radiation.
Lewis in 1926,[156] who mistakenly believed that photons were conserved, contrary to Bose–Einstein statistics; nevertheless the word 'photon' was adopted to express the Einstein postulate of the packet nature of light propagation.
Ultimately, Planck's law of black-body radiation contributed to Einstein's concept of quanta of light carrying linear momentum,[34][132] which became the fundamental basis for the development of quantum mechanics.
It admitted non-linear oscillators as models of atomic quantum states, allowing energetic interaction between their own multiple internal discrete Fourier frequency components, on the occasions of emission or absorption of quanta of radiation.