Poliovirus
Poliovirus, the causative agent of polio (also known as poliomyelitis), is a serotype of the species Enterovirus C, in the family of Picornaviridae.
Because of its short genome and its simple composition—only a strand of RNA and a nonenveloped icosahedral protein coat encapsulating it—poliovirus is widely regarded as the simplest significant virus.
[8][9] Following attachment to the host cell membrane, entry of the viral nucleic acid was thought to occur one of two ways: via the formation of a pore in the plasma membrane through which the RNA is then "injected" into the host cell cytoplasm, or via virus uptake by receptor-mediated endocytosis.
Domain 3 is a self folding RNA element that contains conserved structural motifs in various stable stem loops linked by two four-way junctions.
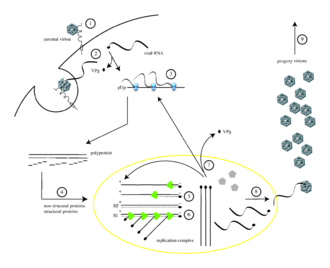
[18]: 165 [19] These individual viral proteins are:[3][20] After translation, transcription and genome replication which involve a single process, synthesis of (+) RNA) is realized.
Which means that VPg is once more utilized as a primer however this time it adds the two uridine triphosphates using a cis-acting replication element (CRE) as a template.
[22][23] The CRE of poliovirus is identified as an unachieved base-paired stem and a final loop consisting of 61 nt.
It is a highly preserved secondary RNA structural element and bedded in the genome's polyprotein-coding region.
Uridylylation process of VPg that takes place at CRE needs the presence of 3CDpro that is an RNA binding protein.
[29][30] Poliovirus is structurally similar to other human enteroviruses (coxsackieviruses, echoviruses, and rhinoviruses), which also use immunoglobulin-like molecules to recognize and enter host cells.
[6] Phylogenetic analysis of the RNA and protein sequences of poliovirus suggests that it may have evolved from a C-cluster Coxsackie A virus ancestor through a mutation in the capsid.
Inactive polio vaccine is prepared by formalin inactivation of three wild, virulent reference strains: Mahoney or Brunenders (PV-1), MEF-1/Lansing (PV-2), and Saukett/Leon (PV-3).
In 95% of cases only a primary, transient presence of viremia (virus in the bloodstream) occurs, and the poliovirus infection is asymptomatic.
In about 5% of cases, the virus spreads and replicates in other sites such as brown fat, reticuloendothelial tissue, and muscle.
The sustained viral replication causes secondary viremia and leads to the development of minor symptoms such as fever, headache, and sore throat.
In cases of paralytic disease, muscle pain and spasms are frequently observed prior to onset of weakness and paralysis.
The first hypothesis predicts that virions pass directly from the blood into the central nervous system by crossing the blood–brain barrier independent of CD155.
First, it can survive the highly acidic conditions of the stomach, allowing ingested viruses to infect the host and spread throughout the body via the lymphatic system.
Polio eradication, the goal of permanent global cessation of circulation of the poliovirus and hence elimination of the poliomyelitis (polio) it causes, is the aim of a multinational public health effort begun in 1988, led by the World Health Organization (WHO), the United Nations Children's Fund (UNICEF) and the Rotary Foundation.
[55] These organizations, along with the U.S. Centers for Disease Control and Prevention (CDC) and The Gates Foundation, have spearheaded the campaign through the Global Polio Eradication Initiative (GPEI).
Nigeria is the latest country to have officially stopped endemic transmission of wild poliovirus, with its last reported case in 2016.
In 1999, the World Health Organization approved the use of the TgPVR mouse as an alternative method of assessing the effectiveness of the vaccine against poliovirus type-3.
[69] A modification of the poliovirus, called PVSRIPO, was tested in early clinical trials as a possible treatment for cancer.
[71][72] A drawback of the attenuated virus used in the Sabin oral polio vaccine is its potential to cause vaccine-associated paralytic poliomyelitis (VAPP) in approximately one individual per every 2.7 million doses administered.
[74] The vaccine derived from this strain, novel oral polio virus type 2 (nOPV2), was granted emergency licencing in 2021, and subsequently full licensure in December 2023.
[79] In 1981, the poliovirus genome was published by two different teams of researchers: by Vincent Racaniello and David Baltimore at MIT[80] and by Naomi Kitamura and Eckard Wimmer at Stony Brook University.
[81] The three-dimensional structure of poliovirus was determined in 1985 by James Hogle at Scripps Research Institute using X-ray crystallography.
[82] In 1981, Racaniello and Baltimore used recombinant DNA technology to generate the first infectious clone of an animal RNA virus, poliovirus.
[83] Creation of the infectious clone propelled understanding of poliovirus biology, and has become a standard technology used to study many other viruses.
In 2002, Eckard Wimmer's group at Stony Brook University succeeded in synthesizing poliovirus from its chemical code, producing the world's first synthetic virus.