RTI-274
RTI(-4229)-274, or 2β-((3,4-Methylenedioxyphenoxy)methyl)-3α-(4-fluorophenyl)nortropane is a phenyltropane homologue of paroxetine developed by the group led by F Ivy Carroll in the 1990s.
RTI decided that they wanted to make all 8 stereoisomers of the phenyltropane paroxetine homolog.
[1] In the case of nocaine it is understood that the SR enantiomer is the one that should be demethylated if it is wanted to improve DAT affinity.
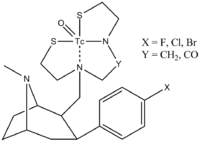
[2] Diagram[dead link] Notice that they are not only interested in ethers, but nitrogen containing Nu's ("TRODAT")[3] The metal is called "Technetium" and is bound by a chelating agent.
To solve the problem of the unexpected aza-bicyclo[3.2.2]nonane rearrangement product, the original synthesis had to be modified as follows;[4] WIN 35428 was N-demethylated and then the NH amine was reacted with a suitable protecting group so that N is no longer nucleophilic.