Ronald Breslow
His seminal contributions include the correct site of reactivity of thiamin diphosphate in enzymes that promote the decarboxylation of pyruvate – based on his pioneering use of proton NMR with small molecule analogues – and the rate enhancement provided by binding to cyclodextrins produced major themes for study in modern organic and biological chemistry.
He also co-discovered the histone deacetylase inhibitor SAHA (Vorinostat) which is FDA-approved for the treatment of cutaneous T-cell lymphoma.
Among Breslow's former Ph.D. students is Robert Grubbs, who won the Nobel Prize in Chemistry in 2005, and Doug La Follette, Secretary of State of Wisconsin.
In 2012, his paper "Evidence for the Likely Origin of Homochirality in Amino Acids, Sugars, and Nucleosides on Prebiotic Earth" was retracted from the Journal of the American Chemical Society due to copyright concerns, leading to a debate on self-plagiarism and the distinction between a personal review and a paper.
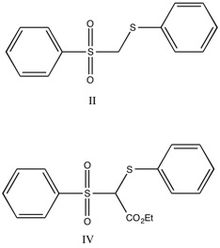
It had been suggested that carbanion-sulfone double bonds will not show aromatic character-primarily as a result of the nodes present in d-orbitals.
[13] Straight chain analogs were chosen based on the comparable acidity, combined with previous studies indicating that steric effects are largely negligible.
There is growing evidence that the chiral preference came from outer space as scientists discovered α-methyl amino acids inside the Murchison meteorite that have a slight enantiomeric excess (ee) for the L conformation.
A common critique is that these amino acids would not be able to tolerate the high temperatures upon entering Earth's atmosphere as the meteorite crashed into the planet.
The most widely accepted theory is that right circularly polarized light in outer space (somewhat) selectively destroyed the D conformation.
However, other astronomers claim that the polarization only occurs in the infrared region, which only has sufficient energy to cause molecular vibrations and stretches-far from being capable of destroying molecules.
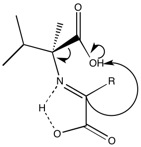
Based on computational calculations, the copper forms a square planar complex (shown below) and sterics facilitate protonation to generate the L amino acid.
As a result, when a slight excess of one enantiomer is present, the ee can be amplified by evaporating solvent-causing the racemate to precipitate.
Researchers have been able to start with an ee of 1% L, and ultimately end up with 95:5 solution of L: D. The results discussed above (particularly the synchrotron argument) led Breslow to propose that the D amino acids and L sugars could generate life in other parts of the universe.

3 H +
3