Silicone rubber
Silicone rubbers are often one- or two-part polymers, and may contain fillers to improve properties or reduce cost.
Silicone rubber is generally non-reactive, stable, and resistant to extreme environments and temperatures from −55 to 300 °C (−70 to 570 °F) while still maintaining its useful properties.
The technically correct term for the various silicone rubbers is polysiloxanes (polydimethylsiloxanes being a large subset), referring to a saturated Si-O backbone.
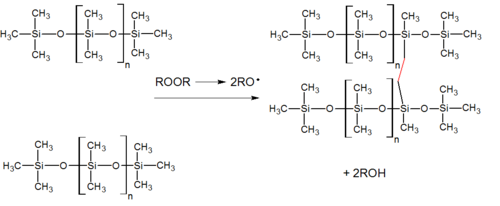
In a platinum-based silicone cure system, also called an addition system (because the key reaction-building polymer is an addition reaction), a hydride- and a vinyl-functional siloxane polymer react in the presence of a platinum complex catalyst, creating an ethyl bridge between the two.
[3] In one-part or RTV (room-temperature vulcanizing) system, a cross-linker exposed to ambient humidity (i.e., water) experiences a hydrolysis step and is left with a hydroxyl or silanol group.
In many cases an additional condensation catalyst is added to fully cure the RTV system and achieve a tack-free surface.
Depending on the type of detached molecule, it is possible to classify silicone systems as acidic, neutral, or alkaline.
[5] Once fully cured, condensation systems are effective as sealants and caulks in plumbing and building construction and as molds for casting polyurethane, epoxy, and polyester resins, waxes, gypsum, and low-melting-temperature metals such as lead.
The glass was very heat resistant, but the phenolic resins would not withstand the higher temperatures that were being encountered in new smaller electric motors.
Silicone rubber is a material of choice in industry when retention of initial shape and mechanical strength are desired under heavy thermal stress or sub-zero temperatures.
Its chemical stability prevents it from affecting any substrate it is in contact with (skin, water, blood, active ingredients, etc.).
Polymer segments in polysiloxanes can move farther and change conformation easily, making for a flexible material.
The difference is partly due to the higher bond energy, the energy required to break the Si-O bond, and also because polyoxymethylene decomposes formaldehyde, which is volatile and escapes driving decomposition forward, but Si-containing decomposition products of silicone are less volatile.
[17] There are many special grades and forms of silicone rubber, including: steam resistant, metal detectable, high tear strength, extreme high temperature, extreme low temperature, electrically conductive, chemical/oil/acid/gas resistant, low smoke emitting, and flame-retardant.
Silicone rubber is available in a range of hardness levels, expressed as Shore A or IRHD between 10 and 100, the higher number being the harder compound.
[18] Once mixed and coloured, silicone rubber can be extruded into tubes, strips, solid cord, or custom profiles according to the size specifications of the manufacturer.
Manufacturers work to set industry tolerances when extruding, cutting, or joining silicone rubber profiles.
Silicone rubber can be 3d printed (liquid deposition modelling LDM) using pump-nozzle extrusion systems.
Unfortunately, standard silicone formulations are optimized to be used by extrusion and injection moulding machines and are not applicable in LDM-based 3D printing.
Liquid silicone rubber is also manufactured for life science applications (syringe pistons, closure for dispensing system, gaskets for IV flow regulator, respiratory masks, and implantable chambers for IV administration), cosmetic products (mascara brush, make-up packaging, make-up applicator, and lipstick moulds), and optics products (circular lens, collimators, Fresnel lenses, and free-form lenses).
As an electrical insulator, silicone rubber has the added virtue of remaining non-conductive when damaged by heat, reducing the likelihood of runaway arcing.