Single-cell analysis
The development of new technologies is increasing scientists' ability to analyze the genome and transcriptome of single cells,[10] and to quantify their proteome and metabolome.
[11][12][13] Mass spectrometry techniques have become important analytical tools for proteomic and metabolomic analysis of single cells.
[14][15] Recent advances have enabled the quantification of thousands of proteins across hundreds of single cells,[16] making possible new types of analysis.
Methods currently used for single-cell isolation include: dielectrophoretic digital sorting, enzymatic digestion, FACS, hydrodynamic traps, laser capture microdissection, manual picking, microfluidics, Inkjet Printing (IJP), micromanipulation, serial dilution, and Raman tweezers.
IJP allows for the adjustment of shear force to the sample ejection, greatly improving cell survivability.
In this technique, the cells or particles are trapped in a particular region for single-cell analysis, usually without application of any external force fields such as optical, electrical, magnetic, or acoustic.
There is a need to explore the insights of SCA in the cell's natural state and development of these techniques is highly essential for that study.
Researchers have highlighted the vast potential field that needs to be explored to develop biochip devices to suit market/researcher demands.
The following principles outline the various microfluidic processes for single-cell separation: droplet-in-oil-based isolation, pneumatic membrane valving, and hydrodynamic cell traps.
Single-cell genomics is heavily dependent on increasing the copies of DNA found in the cell so that there is enough statistical power for accurate sequencing.
The method shown to largely avoid the biases seen in DOP-PCR and MDA is Multiple Annealing and Looping–Based Amplification Cycles (MALBAC).
The main drawback to using MALBAC is that it has reduced accuracy compared to DOP-PCR and MDA due to the enzyme used to copy the DNA.
The first step in quantifying the transcriptome is to convert RNA to cDNA using reverse transcriptase so that the contents of the cell can be sequenced using NGS methods as was done in genomics.
The transcriptome is often used to quantify gene expression instead of the proteome because of the difficulty currently associated with amplifying protein levels sufficiently to make them convenient to study.
[38] These techniques can be highly multiplexed for simultaneous quantification of many targets (panels of up to 38 markers) in single cells.
[45][46] The second generation, SCoPE2,[47][48] increased the throughput by automated and miniaturized sample preparation;[49] It also improved quantitative reliability and proteome coverage by data-driven optimization of LC-MS/MS[50] and peptide identification.
[51] The sensitivity and consistency of these methods have been further improved by prioritization,[52] and massively parallel sample preparation in nanoliter size droplets.
[42][43][44][45][57] These methods are very similar to those used to quantify the proteome of bulk cells, with modifications to accommodate the very small sample volume.
One of the possible ways to measure the content of single cells is nano-DESI (nanospray desorption electrospray ionization).
The two capillaries are touching therefore a liquid bridge can be formed between them and enable the sampling of surfaces as small as a single cell.
Nano-DESI mass spectrometry (MS) enables sensitive molecular profiling and quantification of endogenous species as small as a few hundred fmol-s in single cells in a higher throughput manner.
Lanekoff et al. identified 14 amino acids, 6 metabolites, and several lipid molecules from single cheek cells using nano-DESI MS.[60] In Laser ablation electrospray ionization (LAESI), a laser is used to ablate the surface of the sample and the emitted molecules are ionized in the gas phase by charged droplets from electrospray.
Anderton et al. used this ionization technique coupled to a Fourier transform mass spectrometer to analyze 200 single cells of Allium cepa (red onion) with high spatial resolution.
[62] Pareek et al. performed metabolomics to trace how purines are synthesized within purinosomes and used isotope labeling and SIMS imaging to directly observe hotspots of metabolic activity within frozen HeLa cells.
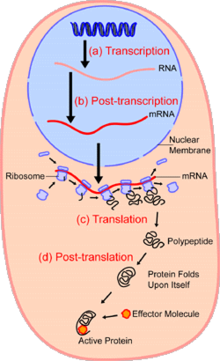
Although transcriptomics has the same purpose as proteomics it is not as accurate at determining gene expression in cells as it does not take into account post-transcriptional regulation (not all messenger RNA transcripts are actually translated into proteins).
Usually these assays use small fluorescent tags attached to molecules of interest, however this has been shown be too invasive for single cell metabolomics, and alters the activity of the metabolites.
Its advantages are that there is no need to develop fluorescent proteins for all molecules of interest, and is capable of detecting metabolites in the femtomole range.
[15] Similar to the methods discussed in proteomics, there has also been success in combining mass spectroscopy with separation techniques such as capillary electrophoresis to quantify metabolites.