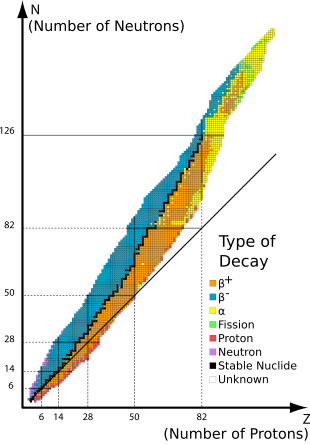Stable nuclide
Stable nuclides are isotopes of a chemical element whose nucleons are in a configuration that does not permit them the surplus energy required to produce a radioactive emission.
If the half-life of a nuclide is comparable to, or greater than, the Earth's age (4.5 billion years), a significant amount will have survived since the formation of the Solar System, and then is said to be primordial.
Many naturally occurring radioisotopes (another 53 or so, for a total of about 339) exhibit still shorter half-lives than 700 million years, but they are made freshly, as daughter products of decay processes of primordial nuclides (for example, radium from uranium), or from ongoing energetic reactions, such as cosmogenic nuclides produced by present bombardment of Earth by cosmic rays (for example, 14C made from nitrogen).
[2] If the predicted half-life falls into an experimentally accessible range, such isotopes have a chance to move from the list of stable nuclides to the radioactive category, once their activity is observed.
Most stable isotopes on Earth are believed to have been formed in processes of nucleosynthesis, either in the Big Bang, or in generations of stars that preceded the formation of the Solar System.
Such nuclei thus instead undergo double beta decay (or are theorized to do so) with half-lives several orders of magnitude larger than the age of the universe.
Odd–odd primordial nuclides are rare because most odd–odd nuclei beta-decay, because the decay products are even–even, and are therefore more strongly bound, due to nuclear pairing effects.
[4] Yet another effect of the instability of an odd number of either type of nucleon is that odd-numbered elements tend to have fewer stable isotopes.
The end of the stable elements occurs after lead, largely because nuclei with 128 neutrons—two neutrons above the magic number 126—are extraordinarily unstable and almost immediately alpha-decay.
A similar phenomenon occurs to a much lesser extent with 84 neutrons—two neutrons above the magic number 82—where various isotopes of lanthanide elements alpha-decay.
The ground state, tantalum-180, is radioactive with half-life 8 hours; in contrast, the decay of the nuclear isomer is extremely strongly forbidden by spin-parity selection rules.
It is expected that improvement of experimental sensitivity will allow discovery of very mild radioactivity of some isotopes now considered stable.
For example, in 2003 it was reported that bismuth-209 (the only primordial isotope of bismuth) is very mildly radioactive, with half-life (1.9 ± 0.2) × 1019 yr,[6][7] confirming earlier theoretical predictions[8] from nuclear physics that bismuth-209 would very slowly alpha decay.
Currently there are 105 "stable" isotopes which are theoretically unstable, 40 of which have been observed in detail with no sign of decay, the lightest in any case being 36Ar.