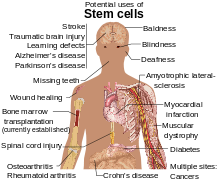
Stem-cell therapy
For over 90 years, hematopoietic stem cell transplantation (HSCT) has been used to treat people with conditions such as leukemia and lymphoma; this is the only widely practiced form of stem-cell therapy.
[8] Another stem-cell therapy, called Prococvhymal, was conditionally approved in Canada in 2012 for the management of acute graft-vs-host disease in children who are unresponsive to steroids.
Research is underway to examine the differentiating capabilities of stem cells found in the umbilical cord, yolk sac and placenta of different animals.
The trial aimed to evaluate the drug GRNOPC1, embryonic stem cell-derived oligodendrocyte progenitor cells, on people with acute spinal cord injury.
[25] In 2013 biotechnology and regenerative medicine company BioTime (AMEX: BTX) acquired Geron's stem cell assets in a stock transaction, with the aim of restarting the clinical trial.
Among them, nanostructured biomaterials are of particular interest because they have the advantage of a high surface-to-volume ratio, and they mimic the physical and biological features of natural ECM at the nanoscale.
[30][31] To further enrich blood supply to the damaged areas, and consequently promote tissue regeneration, platelet-rich plasma could be used in conjunction with stem cell transplantation.
[33][34][35] Preliminary studies related to multiple sclerosis have been conducted,[36][37][38] and a 2020 phase 2 trial found significantly improved outcomes for mesenchymal stem cell treated patients compared to those receiving a sham treatment.
In healthy adult laboratory animals, progenitor cells migrate within the brain and function primarily to maintain neuron populations for olfaction (the sense of smell).
Pharmacological activation of endogenous neural stem cells has been reported to induce neuroprotection and behavioral recovery in adult rat models of neurological disorders.
[44][45][46][38] In 2017, a small-scale study on individuals 60 years or older with aging frailty showed, after intravenous treatment with Mesenchymal stem cells (MSC) from healthy young donors, significant improvements in physical performance measures.
In this process, HSCs are grown together with stromal cells, creating an environment that mimics the conditions of bone marrow, the natural site of red-blood-cell growth.
In 2004, scientists at King's College London discovered a way to cultivate a complete tooth in mice[58] and were able to grow bioengineered teeth stand-alone in the laboratory.
[70] In 2013, scientists have been investigating an alternative approach to treating HIV-1/AIDS, based on the creation of a disease-resistant immune system through transplantation of autologous, gene-modified (HIV-1-resistant) hematopoietic stem and progenitor cells (GM-HSPC).
[76] Furthermore, the trial also did not meet any other secondary MRI endpoints,[77] leading to a conclusion that intracoronary bone marrow stem cell therapy does not offer a functional or clinical benefit.
[79] In 2023, the case of a woman who was infected with Mycobacterium abscessus and sustained meningitis after stem cell treatment for multiple sclerosis at a commercial clinic in Baja California, Mexico was published.
[80] Research conducted on horses, dogs, and cats has led to the development of stem cell treatments in veterinary medicine which can target a wide range of injuries and diseases, such as myocardial infarction, stroke, tendon and ligament damage, osteoarthritis, osteochondrosis and muscular dystrophy, both in large animals as well as in humans.
[81][82][83][84] While investigation of cell-based therapeutics generally reflects human medical needs, the high degree of frequency and severity of certain injuries in racehorses has put veterinary medicine at the forefront of this novel regenerative approach.
Misaligned breaks due to severe trauma, as well as treatments like tumor resections of bone cancer, are prone to improper healing if left to the natural process alone.
Natural healing, guided by the conventional treatments, leads to the formation of fibrous scar tissue that reduces flexibility and full joint movement.
Traditional treatments prevented a large number of horses from returning to full activity and also have a high incidence of re-injury due to the stiff nature of the scarred tendon.
Introduction of both bone marrow and adipose derived stem cells, along with natural mechanical stimulus promoted the regeneration of tendon tissue.
Stem cell treatment not only allowed more horses to return to full duty and also greatly reduced the re-injury rate over a three-year period.
[95] In 2007, a trial was underway for a patch made of a porous substance onto which the stem cells are "seeded" in order to induce tissue regeneration in heart defects.
[98][99][100] In a study to evaluate the treatment of experimentally induced MS in dogs using laser activated non-expanded adipose derived stem cells.
If this becomes a viable option for conservationists, sperm can be produced from high genetic quality individuals who die before reaching sexual maturity, preserving a line that would otherwise be lost.
[101] In the late 1990s and early 2000s, there was an initial wave of companies and clinics offering stem cell therapy, while not substantiating health claims or having regulatory approval.
[102] By 2012, a second wave of companies and clinics had emerged, usually located in developing countries where medicine is less regulated and offering stem cell therapies on a medical tourism model.
In 2018, the FDA sent a warning letter to StemGenex Biologic Laboratories in San Diego, which marketed a service in which it took body fat from people, processed it into mixtures it said contained various forms of stem cells, and administered it back to the person by inhalation, intravenously, or infusion into their spinal cords; the company said the treatment was useful for many chronic and life-threatening conditions.
[109] In 2018, the US Federal Trade Commission found health centers and an individual physician making unsubstantiated claims for stem cell therapies, and forced refunds of some $500,000.