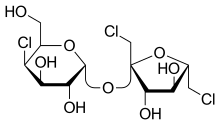
Sucralose
[4] The commercial success of sucralose-based products stems from its favorable comparison to other low-calorie sweeteners in terms of taste, stability, and safety.
[4] Sucralose is used in many food and beverage products because it is a non-nutritive sweetener (14 kilojoules [3.3 kcal] per typical one-gram serving),[3] does not promote dental cavities,[7] is safe for consumption by diabetics and nondiabetics [8] and does not affect insulin levels.
Sucralose mixed with dextrose or maltodextrin (both made from corn) as bulking agents is sold internationally by McNeil Nutritionals under the Splenda brand name.
Sucralose is not hygroscopic when humidity is below 80%, which can lead to baked goods that are noticeably drier and manifest a less dense texture than those made with sucrose.
[14] In some recipes, such as crème brûlée, which require sugar sprinkled on top to partially or fully melt and crystallize, substituting sucralose does not result in the same surface texture, crispness, or crystalline structure.
[16] Various assessments have reported different amounts of maximum acceptable daily intake (ADI), usually measured as mg per kg of body weight.
According to the Canadian Diabetes Association, the amount of sucralose that can be consumed over a person's lifetime without any adverse effects is 9 milligrams per kilogram of body weight per day.
[18][19] In reviewing a 1987 food additive petition by McNeil Nutritionals, the FDA stated that "in the 2-year rodent bioassays ... there was no evidence of carcinogenic activity for either sucralose or its hydrolysis products".
[21] As of 2024[update], reviews of numerous safety and toxicology studies on sucralose concluded that it is not toxic or carcinogenic, even at levels of daily consumption much larger than those typically used.
[39] However, measurements by the Swedish Environmental Research Institute have shown that sewage treatment has little effect on sucralose, which is present in wastewater effluents at levels of several μg/L (ppb).
When heated to very high temperatures (over 350 °C or 662 °F) in metal containers, sucralose can produce polychlorinated dibenzo-p-dioxins and other persistent organic pollutants in the resulting smoke.
[41] Sucralose has been detected in natural waters, but research indicates that the levels found in the environment are far below those required to cause adverse effects to certain kinds of aquatic life.