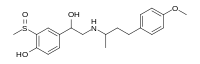
Sulfinalol
[1] The methyl group on a sulfoxide is sufficiently acidic to substitute for phenolic hydroxyl.
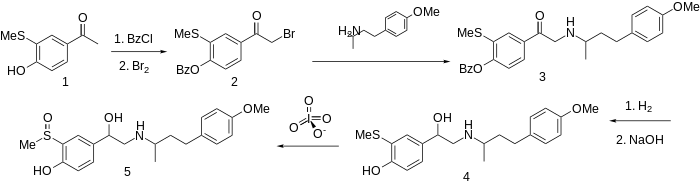
The preparation of this combined α- and β-blocker sulfinalol begins by protection of the phenolic hydroxyl as its benzoate ester.
Successive catalytic reduction and saponification affords aminoalcohol 4.
Oxidation of the sulfide to the sulfoxide with a reagent such as metaperiodate gives sulfinalol (5).
You can help Wikipedia by expanding it.This pharmacology-related article is a stub.