Helium
Helium was first detected as an unknown, yellow spectral line signature in sunlight during a solar eclipse in 1868 by Georges Rayet,[15] Captain C. T. Haig,[16] Norman R. Pogson,[17] and Lieutenant John Herschel,[18] and was subsequently confirmed by French astronomer Jules Janssen.
The formal discovery of the element was made in 1895 by chemists Sir William Ramsay, Per Teodor Cleve, and Nils Abraham Langlet, who found helium emanating from the uranium ore cleveite, which is now not regarded as a separate mineral species, but as a variety of uraninite.
Liquid helium is used in cryogenics (its largest single use, consuming about a quarter of production), and in the cooling of superconducting magnets, with its main commercial application in MRI scanners.
[22] As with any gas whose density differs from that of air, inhaling a small volume of helium temporarily changes the timbre and quality of the human voice.
This radiogenic helium is trapped with natural gas in concentrations as great as 7% by volume, from which it is extracted commercially by a low-temperature separation process called fractional distillation.
[23][24] However, some studies suggest that helium produced deep in the Earth by radioactive decay can collect in natural gas reserves in larger-than-expected quantities,[25] in some cases having been released by volcanic activity.
[33] In 1881, Italian physicist Luigi Palmieri detected helium on Earth for the first time through its D3 spectral line, when he analyzed a material that had been sublimated during a recent eruption of Mount Vesuvius.
[39][40] It was independently isolated from cleveite in the same year by chemists Per Teodor Cleve and Abraham Langlet in Uppsala, Sweden, who collected enough of the gas to accurately determine its atomic weight.
[41][42][28][43] Helium was also isolated by American geochemist William Francis Hillebrand prior to Ramsay's discovery, when he noticed unusual spectral lines while testing a sample of the mineral uraninite.
[69][70] This showed that despite its overall rarity on Earth, helium was concentrated in large quantities under the American Great Plains, available for extraction as a byproduct of natural gas.
[71] Following a suggestion by Sir Richard Threlfall, the United States Navy sponsored three small experimental helium plants during World War I.
For this helium conservation program, the Bureau built a 425-mile (684 km) pipeline from Bushton, Kansas, to connect those plants with the government's partially depleted Cliffside gas field near Amarillo, Texas.
[28][76] The resulting Helium Privatization Act of 1996[77] (Public Law 104–273) directed the United States Department of the Interior to empty the reserve, with sales starting by 2005.
[87] Nasdaq reported (2015) that for Air Products, an international corporation that sells gases for industrial use, helium volumes remain under economic pressure due to feedstock supply constraints.
As in Newtonian mechanics, no system that consists of more than two particles can be solved with an exact analytical mathematical approach (see 3-body problem) and helium is no exception.
All heavier elements (including those necessary for rocky planets like the Earth, and for carbon-based or other life) have thus been created since the Big Bang in stars which were hot enough to fuse helium itself.
For example, in the solar wind together with ionized hydrogen, the particles interact with the Earth's magnetosphere, giving rise to Birkeland currents and the aurora.
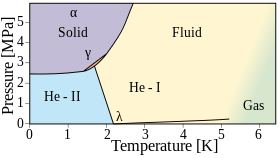
This may be an effect of its boiling point being so close to absolute zero, preventing random molecular motion (thermal energy) from masking the atomic properties.
As with the other noble gases, helium has metastable energy levels that allow it to remain ionized in an electrical discharge with a voltage below its ionization potential.
[30] Helium can form unstable compounds, known as excimers, with tungsten, iodine, fluorine, sulfur, and phosphorus when it is subjected to a glow discharge, to electron bombardment, or reduced to plasma by other means.
Although the helium atoms are not attached by covalent or ionic bonds, these substances have distinct properties and a definite composition, like all stoichiometric chemical compounds.
The resulting crude helium gas is purified by successive exposures to lowering temperatures, in which almost all of the remaining nitrogen and other gases are precipitated out of the gaseous mixture.
Because helium diffuses through solids three times faster than air, it is used as a tracer gas to detect leaks in high-vacuum equipment (such as cryogenic tanks) and high-pressure containers.
It is also used to purge fuel and oxidizer from ground support equipment prior to launch and to pre-cool liquid hydrogen in space vehicles.
[171][172] At depths below 150 metres (490 ft) divers breathing helium-oxygen mixtures begin to experience tremors and a decrease in psychomotor function, symptoms of high-pressure nervous syndrome.
[166] Helium, mixed with a heavier gas such as xenon, is useful for thermoacoustic refrigeration due to the resulting high heat capacity ratio and low Prandtl number.
[177] The use of helium reduces the distorting effects of temperature variations in the space between lenses in some telescopes due to its extremely low index of refraction.
[199] On February 4, 2015, it was revealed that, during the recording of their main TV show on January 28, a 12-year-old member (name withheld) of Japanese all-girl singing group 3B Junior suffered from air embolism, losing consciousness and falling into a coma as a result of air bubbles blocking the flow of blood to the brain after inhaling huge quantities of helium as part of a game.
[200][201] The staff of TV Asahi held an emergency press conference to communicate that the member had been taken to the hospital and is showing signs of rehabilitation such as moving eyes and limbs, but her consciousness has not yet been sufficiently recovered.
[202][203] The safety issues for cryogenic helium are similar to those of liquid nitrogen; its extremely low temperatures can result in cold burns, and the liquid-to-gas expansion ratio can cause explosions if no pressure-relief devices are installed.