Cascade reaction
[3] The main benefits of cascade sequences include high atom economy and reduction of waste generated by the several chemical processes, as well as of the time and work required to carry them out.
[1][3][4] The efficiency and utility of a cascade reaction can be measured in terms of the number of bonds formed in the overall sequence, the degree of increase in the structural complexity via the process, and its applicability to broader classes of substrates.
This increased interest in cascade sequences is reflected by the numerous relevant review articles published in the past couple of decades.
In the cases in which two or more classes of reaction are included in a cascade, the distinction becomes rather arbitrary and the process is labeled according to what can be arguably considered the “major theme”.
[4] In order to highlight the remarkable synthetic utility of cascade reactions, the majority of the examples below come from the total syntheses of complex molecules.
The two nucleophilic attacks occurred predominantly with trans addition to afford intermediate 6, which spontaneously underwent a 4π-conrotatory electrocyclic opening of the cyclobutene ring.
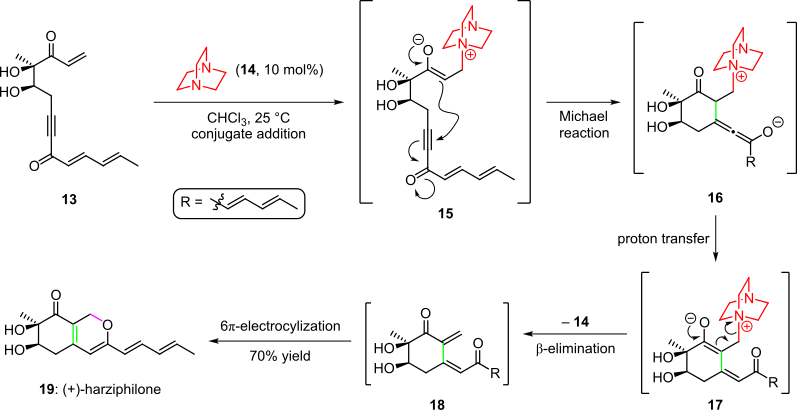
An organocatalytic cascade was employed in the total synthesis of the natural product harziphilone, reported by Sorensen et al. in 2004 (Scheme 3).
Subsequent cyclization by the intramolecular Michael addition of the enolate into the triple bond of the system gave species 16, which afforded intermediate 17 after proton transfer and tautomerization.
The high reactivity of free radical species renders radical-based synthetic approaches decidedly suitable for cascade reactions.
[4] One of the most widely recognized examples of the synthetic utility of radical cascades is the cyclization sequence employed in the total synthesis of (±)-hirsutene, in 1985 (Scheme 6).
[4][19] Herein the highly unsaturated system 39 was first hydrogenated to the conjugated tetraene species 40, which upon heating underwent an 8π-conrotatory electrocyclic ring closure, yielding cyclic intermediate 41.
A second spontaneous electrocyclization, this time a 6π-disrotatory ring closure, converted 41 to the bicyclic species 42, the geometry and stereochemistry of which favored a subsequent intramolecular Diels-Alder reaction.
[4][19] A pericyclic sequence involving intramolecular hetero-cycloaddition reactions was employed in the total synthesis of naturally occurring alkaloid (–)-vindorosine (Scheme 9).
[1][22] In this sequence, a Diels-Alder reaction between 1,2,4,5-hexatetraene 55 and dienophile 56 first formed the highly reactive intermediate 57, which subsequently dimerized to yield [2,2]paracyclophane 58.
[8][23] First, selective rhodium-catalyzed hydroformylation of the less sterically hindered olefin bond in 59 yielded unsaturated aldehyde 60, which under the same conditions was then converted to intermediate 61 via a carbonyl-ene reaction.
[2][24] Treatment of diazoimide 64 with rhodium(II) acetate dimer generated a carbenoid that yielded reactive ylide 65 after an intramolecular cyclization with the neighboring carbonyl group.
Electrophilic opening of the three-membered ring forms cationic species 72, which undergoes a Friedel Crafts-type reaction and then rearomatizes to give tricyclic product 69.
An example of palladium-catalyzed cascades is represented by the asymmetric polyene Heck cyclization used in the preparation of (+)-xestoquinone from triflate substrate 75 (Scheme 16).
A second migratory insertion into the remaining olefin group followed by a β-elimination then occurs to afford product 81 in 82% overall yield and with moderate enantioselectivity.
This multistep tandem reaction greatly simplified the construction of this complex spiroketal structure and eased the path towards the total synthesis of routiennocin.










![Scheme 11. Pericyclic sequence for the synthesis of [2,2]paracyclophanes](http://upload.wikimedia.org/wikipedia/commons/thumb/b/be/Scheme_11_-_peri_-_pcyclophane.svg/681px-Scheme_11_-_peri_-_pcyclophane.svg.png)


![Scheme 14. Gold-catalyzed formal intramolecular [4+2] cycloaddition of 1,6-enynes](http://upload.wikimedia.org/wikipedia/commons/thumb/2/2b/Scheme_14_-_metal_-_gold_enyne.svg/481px-Scheme_14_-_metal_-_gold_enyne.svg.png)
![Scheme 15. Proposed cascade process in the formal intramolecular [4+2] cycloaddition of 1,6-enynes](http://upload.wikimedia.org/wikipedia/commons/thumb/b/ba/Scheme_15_-_metal_-_gold_enyne_mech.svg/751px-Scheme_15_-_metal_-_gold_enyne_mech.svg.png)


