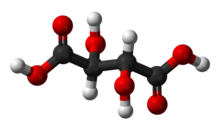
Tartaric acid
[1] Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally in the process of fermentation.
Potassium bitartrate is commonly mixed with sodium bicarbonate and is sold as baking powder used as a leavening agent in food preparation.
[8][9] Louis Pasteur continued this research in 1847 by investigating the shapes of sodium ammonium tartrate crystals, which he found to be chiral.
By manually sorting the differently shaped crystals, Pasteur was the first to produce a pure sample of levotartaric acid.
Tartaric acid in Fehling's solution binds to copper(II) ions, preventing the formation of insoluble hydroxide salts.
In the first step, the maleic acid is epoxidized by hydrogen peroxide using potassium tungstate [de] as a catalyst.
[30] Given this figure, it would take over 500 g (18 oz) to kill a person weighing 70 kg (150 lb) with 50% probability, so it may be safely included in many foods, especially sour-tasting sweets.
The tartrates remaining on the inside of aging barrels were at one time a major industrial source of potassium bitartrate.
For example, it has been used in the production of effervescent salts, in combination with citric acid, to improve the taste of oral medications.
[25] The potassium antimonyl derivative of the acid known as tartar emetic is included, in small doses, in cough syrup as an expectorant.
Grape ingestion commonly leads to gastrointestinal and/or renal issues, with treatment depending on the symptoms; outcomes can vary.