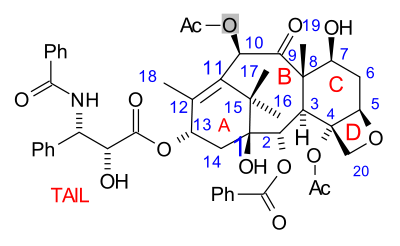
Paclitaxel total synthesis
[1] This diterpenoid is an important drug in the treatment of cancer but, also expensive because the compound is harvested from a scarce resource, namely the Pacific yew (Taxus brevifolia).
Not only is the synthetic reproduction of the compound itself of great commercial and scientific importance, but it also opens the way to paclitaxel derivatives not found in nature but with greater potential.
The anti-tumor activity of a bark extract of the Pacific yew tree was discovered in 1963 as a follow-up of a US government plant screening program already in existence 20 years before that.
In 1990 Bristol-Myers Squibb bought a licence to the patent for this process which in the years to follow earned Florida State University and Holton (with a 40% take) over 200 million US dollars.
What all strategies have in common is synthesis of the baccatin molecule followed by last stage addition of the tail, a process (except for one) based on the Ojima lactam.
The semisynthesis consists of conversion of the amide group to an amine with Schwartz's reagent through an imine followed by acidic workup and a benzoylation.