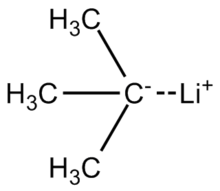
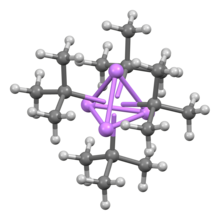
tert-Butyllithium
As an organolithium compound, it has applications in organic synthesis since it is a strong base, capable of deprotonating many carbon molecules, including benzene.
The molecule reacts like a carbanion, as is represented by these two resonance structures:[3] tert-Butyllithium is renowned for deprotonation of carbon acids (C-H bonds).
Air-free techniques are important so as to prevent this compound from reacting violently with oxygen and moisture: The solvents used in common commercial preparations are themselves flammable.
While some researchers take this "pilot light" effect as a sign that the product is "fresh" and has not degraded due to time or improper storage/handling, others prefer to enclose the needle tip or cannula in a short glass tube, which is flushed with an inert gas and sealed at each end with septa.
For example, in 2008 a staff research assistant, Sheharbano Sangji, in the lab of Patrick Harran[15] at the University of California, Los Angeles, died after being severely burned by a fire ignited by tert-butyllithium.