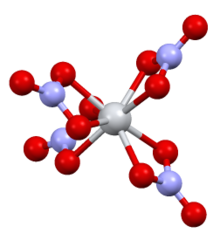
Tin(IV) nitrate
Unlike other nitrates, it reacts with water to produce nitrogen dioxide.
It is structurally very similar to titanium(IV) nitrate, with the only major difference being the Sn–O bond(2.161 Å) being slightly longer than the Ti–O bond(2.068 Å).
Tin(IV) chloride was added to dinitrogen pentoxide at -78 °C, which produced tin(IV) nitrate and nitryl chloride:[4] Attempts to prepare this compound by reacting tin(II) oxide and nitric acid resulted in a formation of tin(II) nitrate hydroxide.
[5] This compound is sensitive to water, it hydrolyzes into tin(IV) oxide and nitrogen dioxide.
Tin(IV) nitrate reacts with trifloroacetic acid anhydride to yield (NO2+)2[Sn(OOCCF3)62−] which is a nitronium salt.