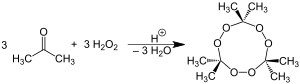
Acetone peroxide
It is produced by the reaction of acetone and hydrogen peroxide to yield a mixture of linear monomer and cyclic dimer, trimer, and tetramer forms.
Acetone peroxide takes the form of a white crystalline powder with a distinctive bleach-like odor when impure, or a fruit-like smell when pure, and can explode powerfully if subjected to heat, friction, static electricity, concentrated sulfuric acid, strong UV radiation, or shock.
[5][6][7] Wolffenstein combined acetone and hydrogen peroxide, and then he allowed the mixture to stand for a week at room temperature, during which time a small quantity of crystals precipitated, which had a melting point of 97 °C (207 °F).
[9][10][11][12][13] Baeyer and Villiger prepared the dimer by combining potassium persulfate in diethyl ether with acetone, under cooling.
[14] They found that the trimer could be prepared by adding hydrochloric acid to a chilled mixture of acetone and hydrogen peroxide.
It is known that traces of sulfuric acid trapped inside the formed acetone peroxide crystals lead to instability.
The authors of the 2004 Dubnikova et al. study confirm that a final redox reaction (combustion) of ozone, oxygen and reactive species into water, various oxides and hydrocarbons takes place within about 180 ps after the initial reaction—within about a micron of the detonation wave.
[26] The tetrameric form of acetone peroxide, prepared under neutral conditions using a tin catalyst in the presence of a chelator or general inhibitor of radical chemistry, is reported to be more chemically stable, although still a very dangerous primary explosive.
[40] Recrystalization of primary explosives may yield large crystals that detonate spontaneously due to internal strain.
For example, triacetone peroxide is the major contaminant found in diisopropyl ether as a result of photochemical oxidation in air.
[45] Numerous methods are used to reduce their appearance, including shifting pH to more alkaline, adjusting reaction temperature, or adding inhibitors of their production.
[60] TATP is attractive to terrorists because it is easily prepared from readily available retail ingredients, such as hair bleach and nail polish remover.
[63][64] Legislative measures to limit the sale of hydrogen peroxide concentrated to 12% or higher have been made in the European Union.