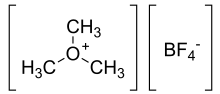
Trimethyloxonium tetrafluoroborate
Trimethyloxonium tetrafluoroborate is the organic compound with the formula [(CH3)3O]+[BF4]−.
[1][a]) This salt is a strong methylating agent, being a synthetic equivalent of CH+3.
It is a white solid that rapidly decomposes upon exposure to atmospheric moisture, although it is robust enough to be weighed quickly without inert atmosphere protection.
[2] Only the exotic dimethylhalonium reagents ([Me2X]+[SbF6]−, X = Cl, Br, I), methyl carboranate reagents, and the transiently-generated methyldiazonium cation (MeN+2) are stronger sources of electrophilic methyl.
Trimethyloxonium tetrafluoroborate is useful for esterification of carboxylic acids under conditions where acid-catalyzed reactions are infeasible: [3]