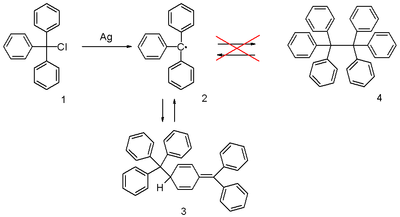
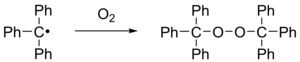Triphenylmethyl radical
[4] When exposed to air, the radical rapidly oxidizes to the peroxide, and the color of the solution changes from yellow to colorless.
[5] Other derivatives of the triphenyl radical with certain substituted phenyl groups do form dimers with a hexaphenylethane-like structure.
Theoretical calculations on a very high level of theory indicate that van der Waals attraction between the tert-butyl groups create a potential minimum that is absent in the unsubstituted molecule.
[6][7] Other derivatives have been reported as the quinoid dimer [8] The class of triaryl-methyl radicals have applications in the synthesis of organic magnets.
[10][11][12] He tried to prepare hexaphenylethane from triphenylmethyl chloride and zinc in benzene in a Wurtz reaction and found that the product, based on its behaviour towards iodine and oxygen, was far more reactive than anticipated.