Vanadium redox battery
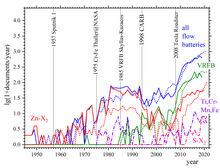
Maria Skyllas-Kazacos presented the first successful demonstration of an All-Vanadium Redox Flow Battery employing dissolved vanadium in a solution of sulfuric acid in the 1980s.
Several 1-5 kW VFB prototype batteries were assembled and field tested in a Solar House in Thailand and in an electric golf cart at UNSW.
[19] The UNSW All-Vanadium Redox Flow Battery patents and technology were licensed to Mitsubishi Chemical Corporation and Kashima-Kita Electric Power Corporation in the mid-1990s and subsequently acquired by Sumitomo Electric Industries where extensive field testing was conducted in a wide range of applications in the late 1990s and early 2000s.
Ammonium and phosphate additives were used to prepare and test a 3 M vanadium electrolyte in a flow cell with excellent results.
[29][30] The pristine carbon-based electrode exhibits hydrophobicity and limited catalytic activity when interacting with vanadium species.
[33] There is currently no consensus regarding the specific functional groups and reaction mechanisms that dictate the interaction of vanadium species on the surface of the electrode.
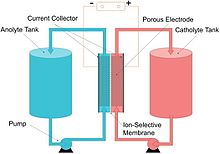
However, vanadium ions can penetrate a PFSA membrane, a phenomenon known as crossing-over, reducing the energy capacity of the battery.
[39][40] Serpentine and interdigitated flow field designs were produced by machining a bipolar plate adjacent to the porous electrode.
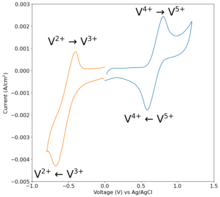
[42][43] The reaction uses the half-reactions:[44] Other useful properties of vanadium flow batteries are their fast response to changing loads and their overload capacities.
This discovery was largely overlooked however and in around 2010 a team from Pacific Northwest National Laboratory proposed a mixed sulfate-chloride electrolyte, that allowed for the use in VRFBs solutions with the vanadium concentration of 2.5 M over a whole temperature range between −20 and +50 °C.
[52] Many researchers explain the increased stability of V(V) at elevated temperatures by the higher proton concentration in the mixed acid electrolyte that shifts the thermal precipitation equilibrium of V(V) away from V2O5.
Nevertheless, because of a high vapor pressure of HCl solutions and the possibility of chlorine generation during charging, such mixed electrolytes have not been widely adopted.
[citation needed] VRFBs' large potential capacity may be best-suited to buffer the irregular output of utility-scale wind and solar systems.
[22] Their reduced self-discharge makes them potentially appropriate in applications that require long-term energy storage with little maintenance—as in military equipment, such as the sensor components of the GATOR mine system.
[56][22] They feature rapid response times well suited to uninterruptible power supply (UPS) applications, where they can replace lead–acid batteries or diesel generators.
These capabilities make VRFBs an effective "all-in-one" solution for microgrids, frequency regulation and load shifting.