Vascular endothelial growth factor
They are important signaling proteins involved in both vasculogenesis (the de novo formation of the embryonic circulatory system) and angiogenesis (the growth of blood vessels from pre-existing vasculature).
It is part of the system that restores the oxygen supply to tissues when blood circulation is inadequate such as in hypoxic conditions.
[4] In 1983 Senger et al. identified a vascular permeability factor secreted by tumors in guinea pigs and hamsters.
[1] In 1989 Ferrara and Henzel described an identical factor in bovine pituitary follicular cells which they purified, cloned and named VEGF.
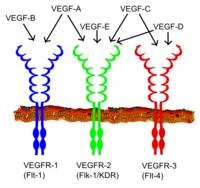
[12] In mammals, the VEGF family comprises five members: VEGF-A, placenta growth factor (PGF), VEGF-B, VEGF-C and VEGF-D.
In addition, inclusion or exclusion of exons 6 and 7 mediate interactions with heparan sulfate proteoglycans (HSPGs) and neuropilin co-receptors on the cell surface, enhancing their ability to bind and activate the VEGF receptors (VEGFRs).
[18] Recently, VEGF-C has been shown to be an important inducer of neurogenesis in the murine subventricular zone, without exerting angiogenic effects.
[19] All members of the VEGF family stimulate cellular responses by binding to tyrosine kinase receptors (the VEGFRs) on the cell surface, causing them to dimerize and become activated through transphosphorylation, although to different sites, times, and extents.
Circulating VEGF-A then binds to VEGF receptors on endothelial cells, triggering a tyrosine kinase pathway leading to angiogenesis.
[clarification needed] The expression of angiopoietin-2 in the absence of VEGF leads to endothelial cell death and vascular regression.
[26] Conversely, a German study done in vivo found that VEGF concentrations actually decreased after a 25% reduction in oxygen intake for 30 minutes.
[28] VEGF-A and the corresponding receptors are rapidly up-regulated after traumatic injury of the central nervous system (CNS).
[25] Although it has not been associated as a biomarker for the diagnosis of acute ischemic stroke,[29] high levels of serum VEGF in the first 48 hours after an cerebral infarct have been associated with a poor prognosis after 6 months[30] and 2 years.
[32] VEGF-A is also released in rheumatoid arthritis in response to TNF-α, increasing endothelial permeability and swelling and also stimulating angiogenesis (formation of capillaries).
VEGF-A plays a role in the disease pathology of the wet form age-related macular degeneration (AMD), which is the leading cause of blindness for the elderly of the industrialized world.
[37] Gene therapies for refractory angina establish expression of VEGF in epicardial cells to promote angiogenesis.