Xenon compounds
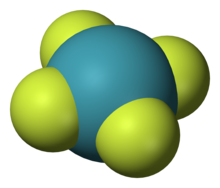
The solid, crystalline difluoride XeF2 is formed when a mixture of fluorine and xenon gases is exposed to ultraviolet light.
[8] Theoretical calculations indicate that the linear molecule XeCl2 is less stable than the van der Waals complex.
[16] Barium perxenate, when treated with concentrated sulfuric acid, yields gaseous xenon tetroxide:[7] To prevent decomposition, the xenon tetroxide thus formed is quickly cooled into a pale-yellow solid.
[21] Xenon can be directly bonded to a less electronegative element than fluorine or oxygen, particularly carbon.
[26] Xenon reversibly complexes gaseous M(CO)5, where M=Cr, Mo, or W. p-block metals also bind noble gases: XeBeO has been observed spectroscopically and both XeBeS and FXeBO are predicted stable.
[29] In 2008, Khriachtchev et al. reported the preparation of HXeOXeH by the photolysis of water within a cryogenic xenon matrix.
[35] Such clathrate hydrates can occur naturally under conditions of high pressure, such as in Lake Vostok underneath the Antarctic ice sheet.
The xenon atom trapped in the fullerene can be observed by 129Xe nuclear magnetic resonance (NMR) spectroscopy.
These observations are not without caveat, however, because the xenon atom has an electronic influence on the reactivity of the fullerene.
[38] When xenon atoms are in the ground energy state, they repel each other and will not form a bond.
When xenon atoms becomes energized, however, they can form an excimer (excited dimer) until the electrons return to the ground state.
The typical lifetime of a xenon excimer is 1–5 nanoseconds, and the decay releases photons with wavelengths of about 150 and 173 nm.
[39][40] Xenon can also form excimers with other elements, such as the halogens bromine, chlorine, and fluorine.