Ytterbium
It is a metal, the fourteenth and penultimate element in the lanthanide series, which is the basis of the relative stability of its +2 oxidation state.
In 1907, the new earth "lutecia" was separated from ytterbia, from which the element "lutecium" (now lutetium) was extracted by Georges Urbain, Carl Auer von Welsbach, and Charles James.
At present, ytterbium is mainly used as a dopant of stainless steel or active laser media, and less often as a gamma ray source.
Natural ytterbium is a mixture of seven stable isotopes, which altogether are present at concentrations of 0.3 parts per million.
This element is mined in China, the United States, Brazil, and India in form of the minerals monazite, euxenite, and xenotime.
[10] Contrary to most other lanthanides, which have a close-packed hexagonal lattice, ytterbium crystallizes in the face-centered cubic system.
Samarium and thulium also behave this way in the +2 state, but europium(II) is stable in aqueous solution.
The main mining areas are China, the United States, Brazil, India, Sri Lanka, and Australia.
[13] As an even-numbered lanthanide, in accordance with the Oddo–Harkins rule, ytterbium is significantly more abundant than its immediate neighbors, thulium and lutetium, which occur in the same concentrate at levels of about 0.5% each.
This is then dissolved using complexing agents, and due to the different types of bonding exhibited by the different lanthanides, it is possible to isolate the compounds.
In the latter method, a buffered acidic solution of trivalent rare earths is treated with molten sodium-mercury alloy, which reduces and dissolves Yb3+.
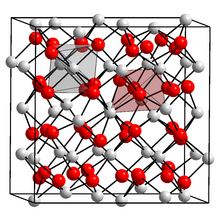
[12] Ytterbium dodecaboride (YbB12) is a crystalline material that has been studied to understand various electronic and structural properties of many chemically related substances.
[29] It is a quantum material; under normal conditions, the interior of the bulk crystal is an insulator whereas the surface is highly conductive.
[30] Among the rare earth elements, ytterbium is one of the few that can form a stable dodecaboride, a property attributed to its comparatively small atomic radius.
[31] Ytterbium was discovered by the Swiss chemist Jean Charles Galissard de Marignac in the year 1878.
[13][27][32][33][34] In 1907, the French chemist Georges Urbain separated Marignac's ytterbia into two components: neoytterbia and lutecia.
The Austrian chemist Carl Auer von Welsbach independently isolated these elements from ytterbia at about the same time, but he called them aldebaranium (Ad; after Aldebaran) and cassiopeium;[13] the American chemist Charles James also independently isolated these elements at about the same time.
Like X-rays, the gamma rays emitted by the source pass through soft tissues of the body, but are blocked by bones and other dense materials.
[41] These clocks developed at the National Institute of Standards and Technology (NIST) rely on about 10,000 ytterbium atoms laser-cooled to 10 microkelvin (10 millionths of a degree above absolute zero) and trapped in an optical lattice—a series of pancake-shaped wells made of laser light.
Another laser that "ticks" 518 trillion times per second (518 THz) provokes a transition between two energy levels in the atoms.
[43] Ytterbium can also be used as a dopant to help improve the grain refinement, strength, and other mechanical properties of stainless steel.
The small quantum defect makes ytterbium a prospective dopant for efficient lasers and power scaling.
At high concentrations, the ytterbium-doped materials show photodarkening[51] (glass fibers) or even a switch to broadband emission[52] (crystals and ceramics) instead of efficient laser action.
This effect may be related with not only overheating, but also with conditions of charge compensation at high concentrations of ytterbium ions.
[53] Much progress has been made in the power scaling lasers and amplifiers produced with ytterbium (Yb) doped optical fibers.
Power levels have increased from the 1 kW regimes due to the advancements in components as well as the Yb-doped fibers.
[54] Ytterbium-doped LMA fibers also have the advantages of a larger mode field diameter, which negates the impacts of nonlinear effects such as stimulated Brillouin scattering and stimulated Raman scattering, which limit the achievement of higher power levels, and provide a distinct advantage over single mode ytterbium-doped fibers.
The charged ion 171Yb+ is used by multiple academic groups and companies as the trapped-ion qubit for quantum computing.
[60] Currently, ytterbium is being investigated as a possible replacement for magnesium in high density pyrotechnic payloads for kinematic infrared decoy flares.
[61] Although ytterbium is fairly stable chemically, it is stored in airtight containers and in an inert atmosphere such as a nitrogen-filled dry box to protect it from air and moisture.