Yttrium(III) chloride
Both are colourless salts that are highly soluble in water and deliquescent.
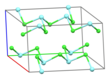
Solid YCl3 adopts a cubic[citation needed] structure with close-packed chloride ions and yttrium ions filling one third of the octahedral holes and the resulting YCl6 octahedra sharing three edges with adjacent octahedra, giving it a layered structure.
[8] These methods produce (NH4)2[YCl5]: The pentachloride decomposes thermally according to the following equation: The thermolysis reaction proceeds via the intermediacy of (NH4)[Y2Cl7].
Treating Y2O3 with aqueous HCl produces the hydrated chloride (YCl3·6H2O).
When heated, this salt yields yttrium oxychloride rather than reverting to the anhydrous form.