Zygomycota
These specialized hyphae usually show negative gravitropism and positive phototropism allowing good spore dispersal.
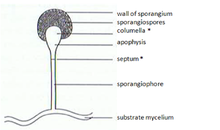
The sporangia wall is thin and is easily destroyed by mechanical stimuli (e.g. falling raindrops, passing animals), leading to the dispersal of the ripe mitospores.
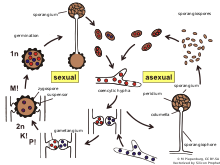
Once two opposite mating types have made initial contact, they give rise to a zygospore through multiple steps.
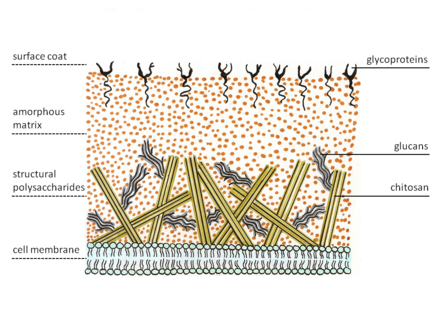
The enzyme on the membrane catalyzes glycosidic bond formations from the nucleotide sugar substrate, uridine diphospho-N-acetyl-D-glucosamine.
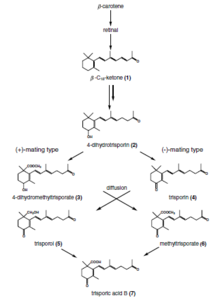
[7] Trisporic acid was discovered in 1964 as a metabolite that caused enhanced carotene production in Blakeslea trispora.
[8] The American mycologist and geneticist Albert Francis Blakeslee discovered that some species of Mucorales were self-sterile (heterothallic), in which interactions of two strains, designated (+) and (-), are necessary for the initiation of sexual activity.
This interaction was found by Hans Burgeff of the University of Goettingen to be due to the exchange of low molecular weight substances that diffused through the substratum and atmosphere.
The elucidation of the hormonal control of sexual interaction in the Mucorales extends over 60 years and involved mycologists and biochemists from Germany, Italy, the Netherlands, the UK and the USA.
[8] Recognition of compatible sexual partners in zygomycota is based on a cooperative biosynthesis pathway of trisporic acid.
In the next stage, septae are established to limit the developing zygospore from the vegetative mycelium and in this way the zygophores become suspensor hyphae and gametangia are formed.
Several cell wall modifications, as well as incorporation of sporopollenin (responsible for the dark colour of spores) take place resulting in a mature zygospore.
Species specificity of these reactions is among others obtained by spatial segregation, physicochemical features of derivatives (volatility and light sensitivity), chemical modifications of trisporoids and transcriptional/posttranscriptional regulation.
An example is the host-parasite interaction of a parasexual nature observed between Parasitella parasitica, a facultative mycoparasite of zygomycetes, and Absidia glauca.
Many morphological similarities in comparison to zygospore formation are seen, but the mature spore is called a sikyospore and is parasitic.
This, coupled with further evidence, has led to the assumption that trisporoids are not strictly species-specific, but that they might represent the general principle of mating recognition in Mucorales.
[9] Light regulation has been investigated in the zygomycetes Phycomyces blakesleeanus, Mucor circinelloides and Pilobolus crystallinus.
For instance, the zygomycota use light as signal to promote vegetative reproduction and growth of aerial hyphae to facilitate spore dispersal.
The product of the gene crgA, which was found in Mucor suppresses the carotene formation by inhibiting the accumulation of carB and carRP mRNAs.
The Zygomycota sporangiophores originate from specialized “basal hyphae” and pass through several distinctive developmental stages until the mature asexual spores are released.
The only model for the mechanism of the gravitropic reaction of Phycomyces is based on the floatability of the vacuole within the surrounding cytoplasm.
[10] The resulting asymmetric distribution of the cytoplasm is proposed to generate increased wall growth on the lower side of horizonally placed sporangiophores as in the thicker cytoplasmic layer forming there the number of vesicles secreting cell-wall material would be higher than on the upper side.
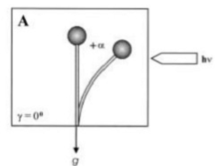
During continuous irradiation with unilateral light, the sporangiophore (fruiting body) of the zygomycete fungus, Phycomyces blakesleeanus reach a bending angle of photogravitropic equilibrium at which the gravitropic and phototropic stimuli balance each other (Fig.
1, bending angle +α, due to light irradiation[11]).In Phycomyces blakesleeanus, wild type sporangiophores contain large, easily seen octahedral paracrystalline crystals with size up to 5×5×5 μm.
These receptors in turn trigger a chain of events which finally leads to the asymmetrical growth of the cell wall.
Most species of Mucor grow rapidly on agar at room temperature filling the Petri dish in 2–3 days with their coarse aerial mycelium.
Growth of Zygomycota in solid agar can produce low or very high fibrous colony that rapidly fills the entire Petri dish.
In its asexual phase it develops bulbous black sporangia at the tips of upright hyphae, each containing hundreds of haploid spores.
Sexual reproduction in Rhizopus stolonifer, as in other zygomycetes, occurs when haploid hyphae of different mating types are in close proximity to each other.
Some zygomycetes disperse their spores in a more precise manner than simply allowing them to drift aimlessly on air currents.
Different mechanisms for forcible spore discharge have evolved among members of the zygomycete order Entomophthorales.