δ34S
[3] That value can be calculated in per mil (‰, parts per thousand) as:[4] Less commonly, if the appropriate isotope abundances are measured, similar formulae can be used to quantify ratio variations between 33S and 32S, and 36S and 32S, reported as δ33S and δ36S, respectively.
[7] During a meeting of the National Science Foundation in April 1962, troilite from the Canyon Diablo meteorite found in Arizona, US, was established as the standard with which δ34S values (and other sulfur stable isotopic ratios) could be calculated.
[6] In 1993, the International Atomic Energy Agency (IAEA) established a new standard, Vienna-CDT (VCDT), based on artificially prepared silver sulfide (IAEA-S-1) that was defined to have a δ34SVCDT value of −0.3‰.
[8] In 1994, the original CDT material was found not to be isotopically homogeneous, with internal variations as great as 0.4‰, confirming its unsuitability as a reference standard.
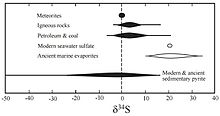
[10][11] The presence of sulfate-reducing bacteria, which reduce sulfate (SO2−4) to hydrogen sulfide (H2S), has played a significant role in the oceanic δ34S value throughout the earth's history.
[7] Archean pyrite found in barite in the Warrawoona Group, Western Australia, with sulfur fractionations as great as 21.1‰ hint at the presence of sulfate-reducers as early as 3,470 million years ago.