Sulfur cycle
In recent times, the large annual input of sulfur from the burning of coal and other fossil fuels has added a substantial amount of SO2 which acts as an air pollutant.
[1] DMS is the largest natural source of sulfur gas, but still only has a residence time of about one day in the atmosphere and a majority of it is redeposited in the oceans rather than making it to land.
[8] These two different regimes appear because at higher temperatures most sulfate-reducing microbes can no longer metabolize due to the denaturation of proteins or deactivation of enzymes,[9] so TSR takes over.
[11] Additionally, the high amounts of hydrogen sulfide found in oil and gas fields is thought to arise from the oxidation of petroleum hydrocarbons by sulfate.
Some PSB can also perform aerobic sulfide oxidation in the presence of oxygen and can even grow chemoautotrophically under low light conditions.
GSB populate stratified lakes with high reduced sulfur concentrations and can even grow in hydrothermal vents by using infra-red light to perform photosynthesis.
[22] The chemical reactions are as follows: In modern oceans, Thiomicrospira, Halothiobacillus, and Beggiatoa are primary sulfur oxidizing bacteria,[22] and form chemosynthetic symbioses with animal hosts.
[25] The Vestimentiferan tube worms that grow around hydrothermal vents lack a digestive tract but contain specialized organelles called trophosomes within which autotrophic, sulfide oxidizing bacteria are housed.
[29] Throughout geologic history the sulfur cycle and the isotopic ratios have coevolved with the biosphere becoming overall more negative with the increases in biologically driven sulfate reduction, but also show substantial positive excursion.
In general positive excursions in the sulfur isotopes mean that there is an excess of pyrite deposition rather than oxidation of sulfide minerals exposed on land.
Dissimilatory sulfate reduction is driven by the degradation of buried organic matter and anaerobic oxidation of methane (AOM) both of which produce carbon dioxide.
[30] Syntrophic aggregates of sulfate reducers and methanotrophs have been discovered and the underlying mechanisms observed include direct interspecies electron transfer using large multi heme complexes.
In the upper sediment layers oxygen and nitrate are the preferred oxidants because of the high energy yield from the reaction, and in the suboxic zones iron and manganese take on the role.
When the oceans condensed on Earth, the atmosphere was essentially swept clean of sulfur gases, owing to their high solubility in water.
[41] Shortly after, at 3.4 Ga the first evidence for minimal fractionation in evaporitic sulfate in association with magmatically derived sulfides can be seen in the rock record.
[43] At 1.8 Ga, Banded iron formations (BIF) are common sedimentary rocks throughout the Archean and Paleoproterozoic; their disappearance marks a distinct shift in the chemistry of ocean water.
It has been hypothesized that BIFs formed during the initial evolution of photosynthetic organisms that had phases of population growth, causing over production of oxygen.
Due to this over production they would poison themselves causing a mass die off, which would cut off the source of oxygen and produce a large amount of CO2 through the decomposition of their bodies, allowing for another bacterial bloom.
[41] Along with the disappearance of BIF, the end of the Paleoproterozoic also marks the first large scale sedimentary exhalative deposits showing a link between mineralization and a likely increase in the amount of sulfate in sea water.
In the Latest Neoproterozoic another major oxidizing event occurred on Earth's surface that resulted in an oxic[check spelling] deep ocean and possibly allowed for the appearance of multicellular life.
[45][46][47][48][49][50][51] Over a shorter time scale (ten million years) changes in the sulfur cycle are easier to observe and can be even better constrained with oxygen isotopes.
By studying oxygen isotopes in ocean sediments over the last 10 million years[52] were able to better constrain the sulfur concentrations in sea water through that same time.
The Great Oxygenation Event (GOE) is characterized by the disappearance of sulfur isotope mass-independent fractionation (MIF) in the sedimentary records at around 2.45 billion years ago (Ga).
Sulfur also acts as a reducing agent in many natural gas reservoirs, and generally, ore-forming fluids have a close relationship with ancient hydrocarbon seeps or vents.
[43] Important sources of sulfur in ore deposits are generally deep-seated, but they can also come from local country rocks, seawater, or marine evaporites.
Ore fluids are generally linked to metal-rich waters that have been heated within a sedimentary basin under elevated thermal conditions, typically in extensional tectonic settings.
[43] Once fossil fuels or precious metals are discovered and either burned or milled, sulfur becomes a waste product that must be dealt with properly, or it can become a pollutant.
The burning of coal, natural gas, and other fossil fuels has greatly increased the amount of sulfur in the atmosphere and ocean and depleted the sedimentary rock sink.
Without human impact sulfur would stay tied up in rocks for millions of years until it was uplifted through tectonic events and then released through erosion and weathering processes.
In the United States, roughly two thirds of all SO2 and one fourth of all NO3 come from electric power generation that relies on burning fossil fuels, like coal.
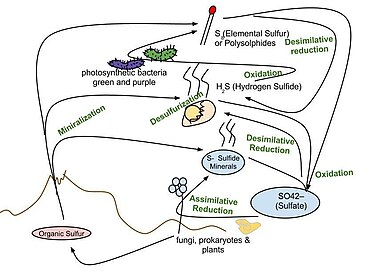

(key intermediate in the sulfur cycle)


