(E)-Stilbene
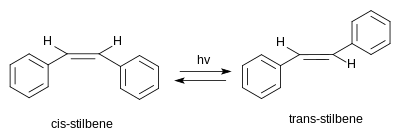
Classified as a diarylethene, it features a central ethylene moiety with one phenyl group substituent on each end of the carbon–carbon double bond.
Trans-stilbene occurs as a white crystalline solid at room temperature and is highly soluble in organic solvents.
[2] The name "stilbene" is derived from the Greek word στίλβω (stilbo), which means "I shine", on account of the lustrous appearance of the compound.
[5] Richard F. Heck[7] and Tsutomu Mizoroki[8] independently reported the synthesis of trans-stilbene by coupling of iodobenzene and styrene using a palladium(II) catalyst, in what is now known as the Mizoroki-Heck reaction.
The achiral meso compound (1R,2S)-1,2-diphenyloxirane arises from cis-stilbene, though peroxide epoxidations of the cis-isomer produce both cis- and trans-epoxide products.
[18][19][20] Bromination of trans-stilbene produces predominantly meso-1,2-dibromo-1,2-diphenylethane (sometimes called meso-stilbene dibromide), in line with a mechanism involving a cyclic bromonium ion intermediate of a typical electrophilic bromine addition reaction;[21] cis-stilbene yields a racemic mixture of the two enantiomers of 1,2-dibromo-1,2-diphenylethane in a non-polar solvent such as carbon tetrachloride, but the extent of production of the meso compound increases with solvent polarity, with a yield of 90% in nitromethane.
[22] The formation of small quantities of the two enantiomers of stilbene dibromide from the trans-isomer suggests that the bromonium ion intermediate exists in chemical equilibrium with a carbocation intermediate PhCHBr–C+(H)Ph with a vacant p orbital vulnerable to nucleophilic attack from either face.
[21] The addition of bromide or tribromide salts restores much of the stereospecificity even in solvents with a dielectric constant above 35.