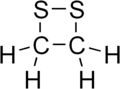1,2-Dithietane
Two sulfur atoms are adjacent, and the molecule is saturated.
The combination of ring strain, and lone pairs of electrons, which repel each other, on the sulfur atoms makes the sulfur-sulfur bond too weak to produce the molecule.
[1][2] 1,2-Dithietan-3-one, the ketone of 1,2-dithietane, was produced in 2008 by reacting α-dithiolactone with ethoxycarbonylformonitrile oxide.
[3] 1,2-Dithietane was claimed to have been made by reacting 1,2-ethanedithiol with iodine, but the major product was an eight-membered ring.
This dithietane polymerised under light, breaking and reforming the S-S bonds to form a long chain -SSCH(OH)CH2-.