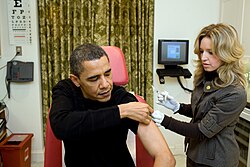
2009 swine flu pandemic vaccine
appeared both effective and safe,[3][4][5][6][7][8][9][10] providing a strong protective immune response and having a similar safety profile to the usual seasonal influenza vaccine.
In some versions of the vaccine used in Europe and Canada, such as Arepanrix and Fluad, an adjuvant is also added, this contains squalene, vitamin E and an emulsifier called polysorbate 80.
[26][27] Prior to the H1N1/09 outbreak, WHO recommended that vaccines for the Northern Hemisphere's 2009–2010 flu season contain an A(H1N1)-like virus, and stocks were made available.
"[34] In fact, a Fairleigh Dickinson University PublicMind poll found in October 2009 that a majority (62%) of New Jerseyans were not planning on getting the vaccine at all.
[36] The global body stated that it wanted companies to donate at least 10% of their production or offer reduced prices for poor countries that could otherwise be left without vaccines if there is a sudden surge in demand.
[37] Gennady Onishchenko, Russia's chief doctor, said on 2 June 2009 that swine flu was not aggressive enough to cause a worldwide pandemic, noting that the current mortality rate of confirmed cases was 1.6% in Mexico and only 0.1% in the United States.
He stated at a press conference, "So far it is unclear if we need to use vaccines against the flu because the virus that is now circulating throughout Europe and North America does not have a pandemic nature."
According to news reports, the WHO's experts would present recommendations to WHO Director-General Margaret Chan, who was expected to issue advice to vaccine manufacturers and the Sixty-second World Health Assembly.
[42] On 20 May 2009, AP reported: "Manufacturers won't be able to start making the [swine flu] vaccine until mid-July at the earliest, weeks later than previous predictions, according to an expert panel convened by WHO.
[45] However, John Sterling, Editor in Chief of Genetic Engineering & Biotechnology News, said on 2 June, "It can take five or six months to come up with an entirely novel influenza vaccine.
[58] Sanofi Pasteur's candidate inactivated H1N1 had several phase II trials planned as of 21 July 2009[update], but had not begun recruiting.
Arepanrix, an AS03-Adjuvanted H1N1 Pandemic Influenza Vaccine similar to Pandemrix and also made by GSK, was authorized by Canada's Minister of Health on 21 October 2009.
[72] Although this trial followed up patients individually, the Government has been criticized for relying on voluntary reporting for post-vaccination evaluation in other circumstances, since this is "unlikely to accurately measure the percentage of people who get adverse effect".
The rate of serious adverse events is one in 200,000 doses distributed, which according to Canada's chief public health officer, is less than expected for the seasonal flu vaccine.
[77] Rare potential adverse events are temporary bleeding disorders and Guillain–Barré syndrome (GBS), a serious condition involving the peripheral nervous system, from which most patients recover fully within a few months to a year.
[63] According to Marie-Paule Kieny of WHO assessing the side-effects of large-scale influenza vaccination is complicated by the fact that in any large population a few people will become ill and die at any time.
[79][80] Chris Shaw, a neuroscientist at the University of British Columbia, expressed concern that serious side-effects may not appear immediately; he said it took five to ten years to see most of the Gulf War syndrome outcomes.
[86] Newsweek states that "wild rumours" about the swine flu vaccine are being spread through e-mails, it writes that "The claims are nearly pure bunk, with only trace amounts of fact.
[87] For example, Newsweek states that some chain e-mails make false claims about squalene (shark liver oil) in vaccines.
The New York Times also notes that anti-vaccine groups have spread "dire warnings" about formulations of the vaccine that contain squalene as an adjuvant.
[74][89] Squalene is a normal part of the human body, made in the liver and circulating in the blood,[90] and is also found in many foods, such as eggs and olive oil.
[91] However, some European and Canadian formulations do contain 25 μg of squalene per dose, which is roughly the amount found in a drop of olive oil.
[99] A 2004 review of the effects of adjuvants on mice and humans concluded that "despite numerous case reports on vaccination induced autoimmunity, most epidemiological studies failed to confirm the association and the risk appears to be extremely low or non-existent", although the authors noted that the possibility that adjuvants might cause damaging immune reactions in a few susceptible people has not been completely ruled out.
[90][105] Multi-dose versions of the vaccine contain the preservative thiomersal (also known as thimerosal), a mercury compound that prevents contamination when the vial is used repeatedly.
[114] The U.K. National Health Service stated in 2003 that "There is no evidence of long-term adverse effects due to the exposure levels of thiomersal in vaccines.
"[115] The World Health Organization concluded that there is "no evidence of toxicity in infants, children or adults exposed to thiomersal in vaccines".
[122][120] Additionally, the woman later said that Jenny McCarthy's anti-vaccine group Generation Rescue had "commandeered my injury to turn it into a poster story for their cause against vaccines.
The company and the Centers for Disease Control and Prevention (CDC) emphasized that the recall was not prompted by safety concerns, and that even though the vaccine is not quite as potent as it is supposed to be, children who received it do not need to be immunized again.
The reports concern children aged 12–16 years where symptoms compatible with narcolepsy, diagnosed after thorough medical investigation, have occurred one to two months after vaccination.
[citation needed] The American Centers for Disease Control and Prevention issued the following recommendations on who should be vaccinated (order is not in priority):[128][129][130][131]