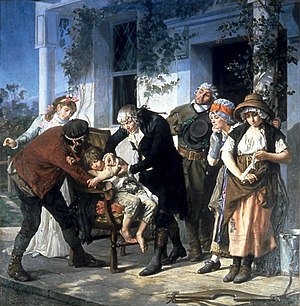Clinical trial
The sponsor designs the trial in coordination with a panel of expert clinical investigators, including what alternative or existing treatments to compare to the new drug and what type(s) of patients might benefit.
[citation needed] As a trial is designed to test hypotheses and rigorously monitor and assess outcomes, it can be seen as an application of the scientific method, specifically the experimental step.
[citation needed] The most common clinical trials evaluate new pharmaceutical products, medical devices, biologics, diagnostic assays, psychological therapies, or other interventions.
Similarly to drugs, manufacturers of medical devices in the United States are required to conduct clinical trials for premarket approval.
[24] Although early medical experimentation was performed often, the use of a control group to provide an accurate comparison for the demonstration of the intervention's efficacy was generally lacking.
[27] John Woodall, an English military surgeon of the British East India Company, had recommended the consumption of citrus fruit from the 17th century, but their use did not become widespread.
[32][33] The English doctor John Haygarth demonstrated the importance of a control group for the correct identification of the placebo effect in his celebrated study of the ineffective remedy called Perkin's tractors.
Among his major ideas include the importance of randomization—the random assignment of individual elements (eg crops or patients) to different groups for the experiment;[35] replication—to reduce uncertainty, measurements should be repeated and experiments replicated to identify sources of variation;[36] blocking—to arrange experimental units into groups of units that are similar to each other, and thus reducing irrelevant sources of variation; use of factorial experiments—efficient at evaluating the effects and possible interactions of several independent factors.
[25] Of these, blocking and factorial design are seldom applied in clinical trials, because the experimental units are human subjects and there is typically only one independent intervention: the treatment.
His certificate for election to the Royal Society called him "... the leader in the development in medicine of the precise experimental methods now used nationally and internationally in the evaluation of new therapeutic and prophylactic agents."
[15] Phase IV trials are performed after the newly approved drug, diagnostic or device is marketed, providing assessment about risks, benefits, or best uses.
[48] In observational studies, the investigators retrospectively assess associations between the treatments given to participants and their health status, with potential for considerable errors in design and interpretation.
[50] Clinical studies can be "sponsored" (financed and organized) by academic institutions, pharmaceutical companies, government entities and even private groups.
[citation needed] A master protocol includes multiple substudies, which may have different objectives and involve coordinated efforts to evaluate one or more medical products in one or more diseases or conditions within the overall study structure.
[citation needed] The protocol describes the scientific rationale, objective(s), design, methodology, statistical considerations and organization of the planned trial.
[citation needed] In any clinical trial, the number of subjects, also called the sample size, has a large impact on the ability to reliably detect and measure the effects of the intervention.
Potential drugs, for example, first have to be discovered, purified, characterized, and tested in labs (in cell and animal studies) before ever undergoing clinical trials.
On average, about eight years pass from the time a cancer drug enters clinical trials until it receives approval from regulatory agencies for sale to the public.
While patient-reported outcome were often paper based in the past, measurements are increasingly being collected using web portals or hand-held ePRO (or eDiary) devices, sometimes wireless.
All studies involving a medical or therapeutic intervention on patients must be approved by a supervising ethics committee before permission is granted to run the trial.
In some cases this can be done, however, for instance, for questions of when to stop sequential treatments (see Odds algorithm), and then quantified methods may play an important role.
[84] Despite explicit recommendations by stakeholders of measures to improve the standards of industry-sponsored medical research,[85] in 2013, Tohen warned of the persistence of a gap in the credibility of conclusions arising from industry-funded clinical trials, and called for ensuring strict adherence to ethical standards in industrial collaborations with academia, in order to avoid further erosion of the public's trust.
At the same time, coordination between Europe, Japan and the United States led to a joint regulatory-industry initiative on international harmonization named after 1990 as the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH)[98] Currently, most clinical trial programs follow ICH guidelines, aimed at "ensuring that good quality, safe and effective medicines are developed and registered in the most efficient and cost-effective manner.
These activities are pursued in the interest of the consumer and public health, to prevent unnecessary duplication of clinical trials in humans and to minimize the use of animal testing without compromising the regulatory obligations of safety and effectiveness.
In the US, sponsors may receive a 50 percent tax credit for clinical trials conducted on drugs being developed for the treatment of orphan diseases.
[108] In later phase trials, subjects may not be paid to ensure their motivation for participating with potential for a health benefit or contributing to medical knowledge.
Small payments may be made for study-related expenses such as travel or as compensation for their time in providing follow-up information about their health after the trial treatment ends.
[114] One recent systematic review of the literature found that race/ethnicity as well as sex were not well-represented nor at times even tracked as participants in a large number of clinical trials of hearing loss management in adults.
[119] The risk information seeking and processing (RISP) model analyzes social implications that affect attitudes and decision making pertaining to clinical trials.
Therefore, the concept of a "decentralized clinical trial" that minimizes or eliminates the need for patients to travel to sites,[125] is now more widespread, a capability improved by telehealth and wearable technologies.