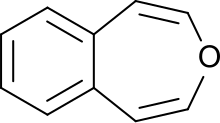
3-Benzoxepin
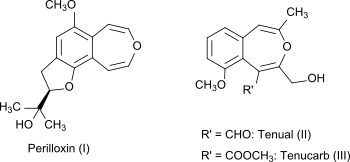
3-Benzoxepin itself is a non-natural compound, but the bicyclic ring system is part of the naturally occurring compounds perilloxin (I) from Perilla frutescens (variant acuta)[4] and tenual (II) and tenucarb (III) from Asphodeline tenuior.
[4] Non-steroidal anti-inflammatory drugs like aspirin and ibuprofen also work by inhibiting the cyclooxygenase enzyme family.
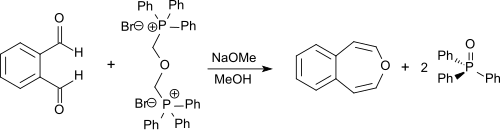
The reaction is performed in dry methanol with sodium methoxide, and the product is obtained in 55% yield.
[8] 3-Benzoxepin is a bright yellow solid that crystallizes in platelets, with a smell similar to naphthalene.
The solid is relatively acid-resistant, only under refluxing in concentrated, acidic alcohol solutions an unsaturated aldehyde is formed (likely an indene-3-aldehyde).